- Chemistry Class 12 Notes
- Physical Chemistry
- Organic Chemistry
- Inorganic Chemistry
- Analytical Chemistry
- Biochemistry
- Chemical Elements
- Chemical Compounds
- Chemical Formula
- Real life Application of Chemistry
- Chemistry Class 8 Notes
- Chemistry Class 9 Notes
- Chemistry Class 10 Notes
- Chemistry Class 11 Notes

Leclanche Cell
Leclanche Cell is a zinc-carbon battery known as a dry cell and is widely used in devices such as flashlights and portable zinc-manganese dioxide systems. It was initially used in telegraphy, signaling, and electric bell work.
In this article, we will see what Leclanche Cell is, its history, construction, applications, etc.
Table of Content
What is Leclanche Cell?
History of leclanche cell, leclanche cell diagram, leclanche cell construction, working of a leclanche cell, difference between leclanche cell and daniel cell, applications of leclanche cells.
Leclanche Cell is a type of battery, a zinc-carbon primary cell that contains an electrolytic solution of ammonium chloride, a carbon cathode, a depolarizer of manganese dioxide, and an anode of zinc. The Leclanché cell is inexpensive to produce and provides a steady flow of electricity for a decent amount of time, making it popular due to its convenience and practicality.
However, more advanced battery technologies, such as alkaline and lithium-ion batteries, have largely replaced it.
Principle of Leclanche Cell
Principle of the Leclanche cell is based on the chemical reaction between the zinc anode and the manganese dioxide cathode in an electrolytic solution of ammonium chloride.
Georges Leclanché, a French engineer, invented the Leclanche cell in 1866. This battery, initially containing a conducting solution of ammonium chloride, a carbon cathode, a manganese dioxide depolarizer, and a zinc anode, quickly succeeded in telegraphy, signaling, and electric bell work.
The cell's design was later adapted to create more portable and efficient dry cells, such as the porous pot cell and the sack cell. Due to its low cost and practicality, it was a commercial success in large sizes and remained the least expensive dry cell for a significant period.
Diagram of Leclanche Cell is shown below:

A Leclanche cell is constructed using a glass vessel filled with electrolyte of ammonium chloride solution. Let's learn in detail about the components of leclanche and steps how to make it.
Components of Leclanche Cell
Materials required to construct a Leclanche cell are as follows:
- Anode: A zinc rod or a cylindrical can be made of a thin sheet of zinc, which serves as the anode and holds all other active and electrolyte materials of the battery
- Cathode: Carbon rod inserted into a porous pot containing a mixture of manganese dioxide and powdered carbon. The cathode is made of carbon mixed with manganese dioxide, which acts as a depolarizer.
- Electrolyte: Ammonium chloride solution, which acts as the conducting solution in the cell
- Porous pot: It contains the cathode and is used to hold the manganese dioxide and powdered carbon mixture.
- Supporting washer: Placed on top of the cathode bobbin.
Steps to Set Up Leclanche Cell
Steps to set up a Leclanche cell are,
Step 1: Take a glass vessel and fill it with ammonium chloride solution, which acts as an electrolyte.
Step 2: Place a zinc rod in the ammonium chloride solution. Zinc rod serves as anode.
Step 3: Fill a porous pot with a mixture of manganese dioxide and powdered carbon. Porous pot contains a carbon rod, which acts as cathode.
Step 4: Insert the porous pot into the glass vessel containing the ammonium chloride solution.
Step 5: Connect the zinc and carbon rods with a wire to complete the circuit.
Step 6: Leclanche cell is now set up and ready to use.
Note: Leclanche cell can also be set up in a dry cell form, which uses a paste electrolyte instead of a liquid one.
The working of leclanche cell can be understood in the terms of following chemical reactions:
- Zinc particles on the surface of the anode oxidize, releasing electrons and forming zinc ions in the electrolyte solution according to the following reaction:
Zn (s) → Zn 2+ (aq) + 2e −
- Released electrons flow through the external circuit and reach the cathode.
- At cathode, the ammonium ions in the electrolyte solution are reduced to molecular hydrogen and ammonia gas according to the following reaction:
2NH 4 + (aq) + 2e − → 2NH 3(g) + H 2(g)
- Manganese dioxide in the cathode acts as a depolarizer, which absorbs the hydrogen ions and prevents the hydrogen gas from forming a layer on the cathode surface. The absorbed hydrogen ions react with the manganese dioxide to form water and manganese oxide according to the following reaction:
MnO 2(s) + 2NH 4 + (aq) + 2e − → MnO(OH) (s) + 2NH 3(g ) + H 2 O (l)
- Overall reaction taking place in the Leclanche cell is:
Zn (s) + 2MnO 2(s) + 2NH 4 Cl (aq) → ZnCl 2(aq) + Mn 2 O 3(s) + 2NH 3(g) + H 2 O (l)
- Difference in the concentration of the zinc and hydrogen ions between the anode and cathode creates a potential difference, resulting in voltage production.
- Leclanche cell produces a voltage of around 1.5 volts and is commonly used in signaling networks and systems where a low current value is needed.
Difference between Leclanche Cell and Daniel Cell is given below in table.
Some of applications of Leclanche cell are,
- Signaling Networks: Leclanche cell is widely used in signaling networks due to its ability to provide a steady flow of electricity for a decent amount of time
- Telegraphy: Leclanche cell was initially used in telegraphy, where it provided a voltage of 1.4 volts, making it suitable for transmitting electrical signals over long distances
- Early Telephones: Dry cell form of the Leclanche cell was used to power early telephones, usually from an adjacent wooden box affixed to the telephone
- Electric Bells: Leclanche cell was also used in electric bell work, where it provided a steady flow of electricity to power the bells
Electric Cell Electrochemical Cell Galvanic Cell
Leclanche Cell Frequently Asked Questions
How does leclanche cell work.
Leclanche cell works through a chemical reaction between zinc and manganese dioxide, with an ammonium chloride solution acting as the electrolyte.
What is Voltage Produced by Leclanche Cell?
Voltage produced by a Leclanche cell is around 1.5 volts.
What is Construction of Leclanche Cell?
Construction of a Leclanche cell includes a zinc anode, a manganese dioxide cathode, and an ammonium chloride electrolyte in a porous pot.
What are Uses of Leclanche Cell?
Leclanche cells are commonly used in devices like flashlights and portable electronic gadgets.
How is Leclanche Cell different from Daniel Cell?
Unlike Daniel cell, a Leclanche cell is dry, making it more portable and suitable for everyday devices, while a Daniel cell uses a liquid electrolyte.
What is Leclanche Cell also Called?
Leclanche Cell is also called as Zinc Carbon Battery.
What is Example of Leclanche Cell?
Leclanche Cell is also called Dry Cell.
Similar Reads
- Leclanche Cell Leclanche Cell is a zinc-carbon battery known as a dry cell and is widely used in devices such as flashlights and portable zinc-manganese dioxide systems. It was initially used in telegraphy, signaling, and electric bell work. In this article, we will see what Leclanche Cell is, its history, constru 6 min read
- Cell Membrane The cell membrane, also known as the plasma membrane, bounds the cell. The cell membrane is composed of proteins and lipids. It is a selectively permeable membrane that binds the cell and separates the cell from the outside environment. In this article, we will study cell membrane structure, functio 8 min read
- Galvanic Cell Galvanic Cell also called Voltaic Cell is an electrochemical device that converts spontaneous chemical energy generated in a redox reaction into electrical energy. Table of Content What is Galvanic Cell?Cell DefinitionElectrolytic Cell DefinitionElectrochemical Cell DefinitionPrimary Cell & Seco 12 min read
- Electric Cell An electric cell is an electrical power supply that generates electrical energy from chemical reactions, it is simply a device that is used to maintain the electric current. The electric cell was invented by Alessandro Volta in 1799. Each cell consists of two ends called terminals. The metal cap on 13 min read
- Electrochemical Cells Electrochemical Cell is a device that may either generate electrical energy from chemical reactions or use electrical energy that is supplied to it to speed up chemical reactions. There are various types of electrochemical cells and they are used in our daily activities such as cells that are used i 12 min read
- Cell Wall Cell wall is the non-living material that protects a cell's outermost layer. It might be firm, elastic, or periodically rigid. It serves as a filtration system as well as structural support and protection for the cell. Cell walls are absent in many eukaryotes, including animals, but they are present 7 min read
- Types of Electric Cell Electric cell is the basic component of the electronics industry and is used everywhere as a power supply. Batteries that are used for large voltage supply are also made up of a collection of electric cells. In this article, We will be going through what is the Electric cell, we will Look at its typ 9 min read
- What is a Cell in Excel? MS-EXCEL is a part of Microsoft Office suite software. It is an electronic spreadsheet with multiple rows and columns, used for organizing data and performing different calculations. A spreadsheet takes the shape of a table, consisting of rows and columns. A cell is created at the intersection point 5 min read
- Mercury Cell Mercury Cell is a type of Primary Cell, which is non-rechargeable in nature, meaning it can only be used once before discarding it. The Mercury Cell is generally a small button-like structure and is mainly used in low-current devices such as watches, BIOS batteries on motherboards, and pacemakers. T 7 min read
- Eukaryotic Cells Eukaryotic cells include all the protists, plants, animals, and fungi. It forms the domain Eukaryota and possesses an organized nucleus with a nuclear envelope. Their genetic material is organized into chromosomes. Eukaryotes contain membrane-bound organelles like Golgi apparatus, mitochondria, endo 7 min read
- Cell Potential Cell Potential is the difference in electrical potential between an electrochemical cell's two electrodes. An electrochemical cell is a device that uses an electrochemical reaction to transform chemical energy into electrical energy. The differential in electron affinities between the two electrodes 8 min read
- Single Cell Protein (SCP) Single Cell Protein (SCP) CBSE Class 12- Strategies For Enhancement in Food Production: Single Cell Protein (SCP) refers to a type of protein-rich biomass produced from microorganisms, such as bacteria, algae, fungi, or yeast, through fermentation or other biotechnological processes. Single Cell Pro 5 min read
- Diagram of Cell Membrane The diagram of cell membrane shows that the cell membrane consists of a semipermeable lipid bilayer. The function of cell membrane is to regulate the transport of materials entering and exiting the cell. The diagram of cell membrane in cross-section shows that the main components are phospholipids, 4 min read
- Crystal Lattice and Unit Cell In crystalline solids, their constituent particles have a definite arrangement in three dimensions. The positions of these particles in the crystal relative to each other are usually represented by points. The dispensation of these unendurable sets of points is called a space lattice. The positions 7 min read
- Cellular Respiration Cellular Respiration is a vital process that occurs in living things. It is a process by which cells turn nutrients into adenosine triphosphate (ATP), which is their source of energy. Glycolysis, the Krebs cycle (also referred to as the citric acid cycle or tricarboxylic acid cycle), and oxidative p 8 min read
- Diagram of Plant Cell Diagram of Plant Cell illustrates that every plant cell is protected by a cell wall that helps in maintaining its shape. They are eukaryotic cells having a true nucleus and specialized structures termed organelles, both of which perform specific roles essential for the survival of the cell. Diagram 4 min read
- Diagram of Cell Cycle The diagram of cell cycle provides insight into the essential process that all cells undergo to grow, replicate, and generate new cells. A cell cycle showcases a continuous sequence crucial for maintaining organism function and growth. The diagram of cell cycle class 11 is an important diagram which 5 min read
- Dry Cell Dry Cell is a portable electrochemical cell invented by German scientist Carl Gassner in 1888. Unlike traditional wet cells, a dry cell features a paste or gel-like electrolyte, eliminating the risk of leakage and enhancing portability. It is commonly used in household essentials such as flashlights 12 min read
- Cell Wall Diagram The cell wall diagram illustrates its complex structure and diverse functions. Cell walls provide structural support, and protection, and regulate cell processes. The cell wall diagram class 9 and 11 is an important diagram that is often asked in the examinations. The cell wall is made up of primari 4 min read
- School Chemistry
- School Learning
- Chemistry-Class-12
- Electrochemistry
Improve your Coding Skills with Practice
What kind of Experience do you want to share?

Leclanche Cell Diagram & Working
The Leclanche cell is a battery which is named after the French scientist Georges Leclanché who invented it in 1866. The Leclanche cell e.m.f. is 1.5 volt. The application of the Leclanche cell was in electric bells, signalling, and telegraphy.
Construction
Leclanche Cell consists of a glass vessel containing a saturated solution of ammonium chloride (NH 4 Cl) as shown in figure 1. An amalgamated zinc rod ( impure zinc rod covered with a layer of mercury ) and a porous pot containing a carbon rod packed in a mixture of manganese dioxide (MnO 2 ) and powered coke are placed in the solution of NH 4 Cl.
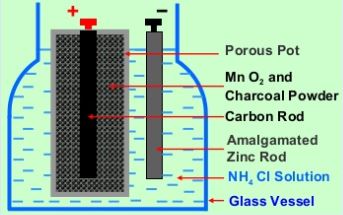
The porous pot is sealed at the top with pitch. Zinc rod forms the negative electrode and the carbon rod forms the positive electrode of the cell. Here MnO 2 acts as a depolariser and powered coke makes MnO 2 electrically conducting.
Anode (negative electrode): Zinc (Zn) rod
Cathode (positive electrode): Carbon (C) rod
Depolariser: Manganese dioxide (MnO 2 )
Electrolyte: Ammonium chloride (NH 4 Cl)
Leclanche Cell Working
When zinc and carbon rods are connected to the external load, the following reactions take place in the cell.
(a). Zn → Zn ++ + 2e −
(b). 2NH 4 Cl → 2NH 3 ↑ + 2H + + 2Cl −
(c). Zn ++ + 2Cl − → ZnCl 2
combining equations (a), (b), and (c), we get
Zn + 2NH 4 Cl → ZnCl 2 + 2NH 3 ↑ + 2H + + 2e −
The negative charge (2e − ) released is collected by the zinc rod and it becomes negatively charged. Ammonia gas (2NH 3 ↑) escapes.
The hydrogen ions (2H + ) diffuse through the porous pot and react with MnO 2 as follows
2H + + 2MnO 2 → Mn 2 O 3 + H 2 O + 2e +
The positive charge (2e + ) is acquired by the carbon rod which becomes positively charged. Mn 2 O 3 formed in the porous pot is again converted into MnO 2 by absorbing oxygen (O 2 ) from the air when left to itself for some time.
The potential difference between the negatively charged zinc rod (anode) and positively charged carbon rod (cathode) is set up. The e.m.f. of Leclanche cell is 1.5 volt.
Limitations
1. The internal resistance of this cell is high.
2. This cell can not supply continuous current for a long time but is used only for intermittent works.
Frequently Asked Questions
Q1. Why can’t we use Leclanche cell in an experiment in which continuous and constant supply of current is required?
Answer. we can’t use Leclanche cell in an experiment in which continuous and constant supply of current is required because its e.m.f. decreases with the passage of time and hence cannot supply constant current for long time.
Q2. What do you mean by amalgamated zinc rod?
Answer. The impure zinc rod covered with a layer of mercury is called amalgamated zinc rod.
Q3. Name the electrolyte used in the Leclanche cell.
Answer. The electrolyte used in the Leclanche cell is Ammonium chloride (NH 4 Cl).
Q4. Name the depolariser used in the Leclanche cell.
Answer. The depolariser used in the Leclanche cell is Manganese dioxide (MnO 2 ).
- Daniell Cell Diagram & Working
- Beat Frequency Oscillator Working & Limitations
- Photoelectric Cell – Types & Applications
- Ionization Chamber | Working
- Fuel Cell – Working & Applications
- Half Subtractor – Truth table & Logic Diagram
- Full Subtractor – Truth table & Logic Diagram
- Screw Gauge – Working & Diagram
- Digital Frequency Meter – Block Diagram & Working
- What is an Operational Amplifier (Op-amp)? Working, Pin-Diagram & Applications
Leave a Comment Cancel reply
This site uses Akismet to reduce spam. Learn how your comment data is processed .
Skip to main content
Physics Museum
- Search Catalogue
- Director's Blog
- Multimedia Gallery

350 - Leclanche Cells

Two Glass square-section vessels with a circular openings on top. Base of vessels 9.5*9.5, height 16, total height 20. F2/4 embossed in base of one. Ceramic pots 7cm diameters.
Invented by French scientist Georges Leclanche (1839-1882) in 1866, this battery in its usual form uses a central carbon electrode surrounded by manganese dioxide in a porous (old form) or agglomerate carbon blocks (new form), with zinc rod for the positive terminal placed in a solution of ammonium chloride (sal ammoniac) in a glass bottle outside the central electrode. To maintain the efficiency of the cell it should be placed in a cool dry situation, a little water added occasionally to compensate for the evaporation of the liquid and at intervals, a little sal ammoniac. With a little rest, any accumulation of hydrogen becomes oxidised and the cell recovers its power.
The e.m.f. of a Leclanche cell is about 1.5 volts buts its resistance may amount to several ohms when a porous pot is employed. It was used extensively for telegraphy, signalling and electric bell work; and for most work where intermittent current is required and where it is essential that the battery should require very little attention from time to time.

On This Site
- History of Physics at UQ
- The International Year of Light Tour
- 2014 - International Year of Crystallography
+61 7 3365 1111
Other Campuses: UQ Gatton , UQ Herston
Maps and Directions
© 2024 The University of Queensland
A Member of
Privacy & Terms of use | Feedback
ABN : 63 942 912 684 CRICOS Provider No: 00025B
Quick Links
- Emergency Contact
Social Media
- Giving to UQ
- Faculties & Divisions
- UQ Contacts
Ph. 3365 3333
CBSE Class 12 Physics Practical Section-A Exp-3-(i)
Section – a experiment -3, objective: to verify the laws of combination (series) of resistances by using a meter bridge ., required apparatus:.
A metre bridge, a Leclanche cell (battery eliminator), a galvanometer, a resistance box, a jockey, two resistance wires or two resistance coils of known resistances, a set square, connecting wires, a piece of sandpaper.
Formula Used:
(i) The resistance r of a resistance wire or coil is given by
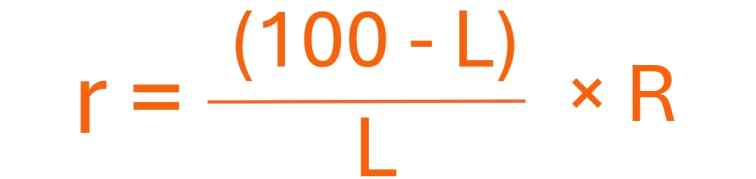
Where, R is known resistance placed in the left gap and unknown resistance r in the right gap of the metre bridge. I cm is the length of a metre bridge wire from zero end up to the balance point.
(ii) When r1 and r2 are connected in series, then their combined resistance.

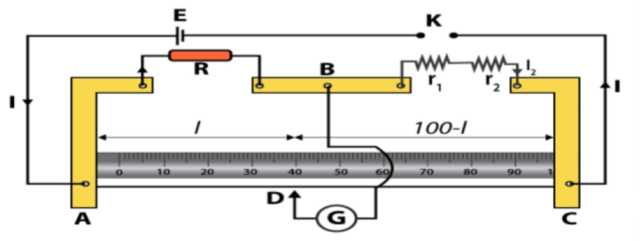
Circuit Diagram:
Observations Table :
Table for unknown resistance
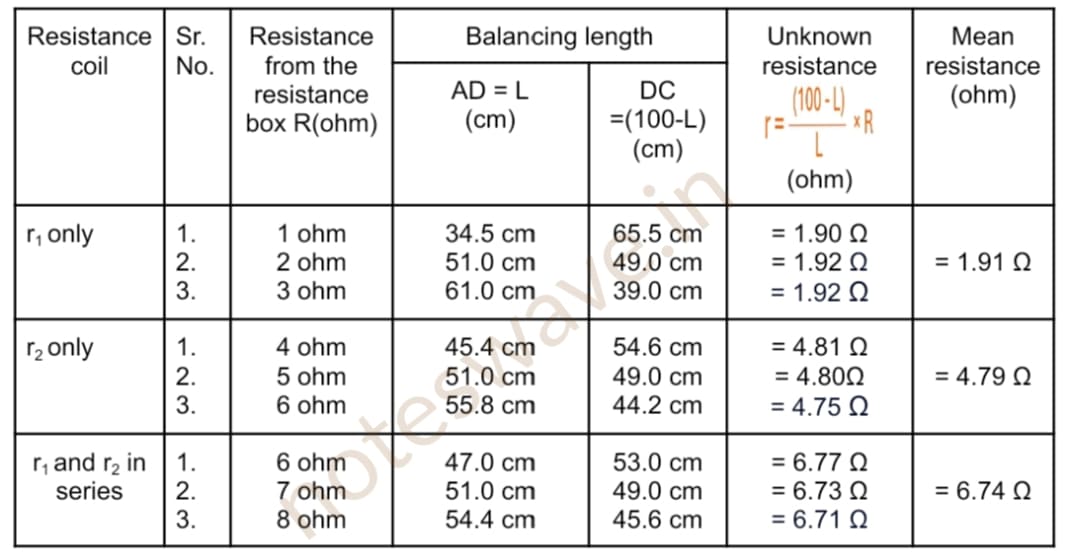
Calculations:
The experimental value of Rs = 6.74 Ω
Theoretical value of Rs = r1 + r2 = 1.91 Ω + 4.79 Ω = 6.70 Ω
Difference ( if any ) = 6.74 Ω – 6.70 Ω = 0.04 Ω
Within the limits of experimental error, experimental and theoretical values of R are the same. Hence, the law of resistance in series is verified.
Precautions:
1. The connections should be neat, tight and clean.
2. Plugs should be tightly connected in the resistance box.
3. The movement of the jockey should be gentle and it shouldn’t be rubbed.
4. The key K should be inserted only when the observations are to be taken.
5. The null point should be between 30 cm and 70 cm.
6. To avoid the error of parallax, the set square should be used to note the null point.
7. There shouldn’t be any loops in the wire.
Sources Of Error:
1. The screws of the instrument might be loose.
2. The plugs may not be clean.
3. The wire might be of non-uniform diameter.
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Leclanché-Element
- Primärbatterie
- Historische Batterie
Das Leclanché-Element ist ein historisches galvanisches Element , das von Georges Leclanché entwickelt und 1866 patentiert wurde. [1] Es stellt eine elektrische Batterie (Primärelement) dar und war in der ursprünglichen Form mit flüssigem Elektrolyt ausgestattet. Es zählt damit zu den heute nicht mehr verwendeten „Nassbatterien“. Verbesserungen führten zu einem gelierten Elektrolyt und es stellt einen Vorläufer der Trockenbatterien wie dem Zink-Kohle-Element und der Alkali-Mangan-Batterie dar.
Allgemeines

Das Leclanché-Element weist eine Klemmenspannung von 1,5 V auf und besteht aus einer Anode aus Zink , die den negativen Anschluss darstellt, einem Elektrolyt aus Ammoniumchlorid , und einer Kathode aus Graphit , die den positiven Anschluss der Zelle darstellt. Die Kathode ist zum Elektrolyt hin durch Mangandioxid (Braunstein) umgeben, der als Depolarisator wirkt.
Das Leclanché-Element war wirtschaftlich über viele Jahrzehnte erfolgreich und wurde unter anderem zur Versorgung von Eisenbahntelegraphen und Hausklingeln eingesetzt. Dabei durchlief das Element über Jahre laufende Verbesserungen: Eine wesentliche Verbesserung und erster Schritt zum Trockenelement erfolgte durch den Ersatz des flüssigen Elektrolyts durch mit Ammoniumchlorid getränktes Weizenmehl. In weiterer Folge wurde das gelierte Elektrolyt durch dünne Separatorpapiere in Sektoren aufgeteilt und durch Beigabe von Zinkchlorid die Energiedichte erhöht. Im Bereich der Zinkanode kamen verschiedene Metalllegierungen und Verschlusssysteme zum Einsatz, um die Wasserstoffentwicklung bei der Entladung zu reduzieren bzw. durch Luftabschluss die Lagerfähigkeit der Elemente zu erhöhen.
Elektrochemie

Die Reaktionsgleichung bei Entladung der Zelle lautet:
Negative Elektrode ( Anode ):
Positive Elektrode ( Kathode ):
Elektrolytlösung ( Komplexbildung ):
Als Gesamtreaktion ergibt sich:
Literaturquellen
- Lucien F. Trueb, Paul Rüetschi: Batterien und Akkumulatoren . 1. Auflage. Springer, 1998 , ISBN 3-540-62997-1.
- H.A. Kiehn: Battery Technology Handbook . 2. Auflage. Marcel Dekker Inc., 2003 , ISBN 0-8247-4249-4.
Einzelnachweise
- ↑ Georges Leclanché: Französisches Patent Nr. 71 865, erteilt 1866
Primärzellen : Alkali-Mangan-Batterie | Lithiumbatterie | Lithium-Eisensulfid-Batterie | Lithium-Mangandioxid-Batterie | Lithium-Thionylchlorid-Batterie | Lithium-Schwefeldioxid-Batterie | Lithium-Kohlenstoffmonofluorid-Batterie | Nickel-Oxyhydroxid-Batterie | Quecksilberoxid-Zink-Batterie | Silberoxid-Zink-Batterie | Zink-Kohle-Zelle | Zinkchlorid-Batterie | Zink-Luft-Batterie Sekundärzellen : Bleiakkumulator | Natrium-Schwefel-Akkumulator | Nickel-Cadmium-Akkumulator | Nickel-Eisen-Akkumulator | Nickel-Lithium-Akkumulator | Nickel-Metallhydrid-Akkumulator | Nickel-Wasserstoff-Akkumulator | Nickel-Zink-Akkumulator | Lithium-Eisenphosphat-Akkumulator | Lithium-Ionen-Akkumulator | Lithium-Mangan-Akkumulator | Lithium-Polymer-Akkumulator | Lithium-Schwefel-Akkumulator | Silber-Zink-Akkumulator | STAIR-Zelle | Vanadium-Redox-Akkumulator | Zink-Brom-Akkumulator | Zink-Luft-Akkumulator | Zebra-Batterie | Zellulose-Polypyrrol-Zelle | Zinn-Schwefel-Lithium-Akkumulator Historische Zellen : Daniell-Element | Gravity-Daniell-Element | Leclanché-Element | Voltasche Säule | Clark-Normalelement | Weston-Normalelement | Zambonisäule Ausführungen: Akkumulator | Batterie | Brennstoffzelle | Knopfzelle | Konzentrationselement | Redox-Flow-Zelle | Thermalbatterie Bestandteile: Halbzelle ( Donator- und Akzeptorhalbzelle )
Everything you want to know about batteries, and more!
Leclanché cell – What is it?
Leclanche cell is a primary cell, handy for sporadic use, with positive anode of zinc encompassed by a mixture of manganese dioxide and powdered carbon in a pot, which is porous. The pot and the negative zinc terminal remained in a container holding ammonium chloride solution. The electromotive force (emf) is nearly 1 -4 volt.
Going back to its history, the Leclanche cell was invented by the French engineer Georges Leclanche in 1866. Leclanche’s battery, additionally called a zinc-carbon battery, made use of an alternate type of cell than its antecedents. Rather than lead, the French engineer utilized zinc and a carbon-manganese dioxide mixture for his terminals. He additionally made use of ammonium chloride instead of the sulfuric acid that had been used as the preferred electrolyte . The changes he made to his battery made the cell less dangerous and lighter than the most commonly used Plante model.
Types of Leclanche’s cell include:
a) zinc (Carbon cathode) b) zinc chloride (Ammonium chloride electrolyte reinstated by zinc chloride) c) alkaline manganese (Ammonium chloride terminal displaced by potassium hydroxide)
How does it work?
The process which generates power in a Leclanché cell starts when zinc particles on the surface of the anode oxidize, i.e. when zinc atoms surrender their valence electrons to end up becoming the positively charged particles. The zinc particles move far from the anode while leaving their electrons on its surface that makes the anode more negatively charged than the cathode. At the point when the cell is associated in an outer electrical circuit, the excess electrons on the zinc anode gush through the circuit to the cathode made up of carbon. This flow of electrons frames the electric current.
After going through the entire circuit, when electrons enter the carbon rod, which is the cathode, they join together with water and MNO2 (Manganese Dioxide) that further reacts with each other to produce negatively charged hydroxide ions and manganese oxide(Mn2O3). Whole of this process is accompanied by secondary reaction, wherein the negative hydroxide ions react with positive ammonium ions in the electrolyte of ammonium chloride to produce molecules of water and ammonia.
Practical Use
The Leclanche cell was utilized widely for telegraphy, electric bell and signaling work; and for work where intermittent and low current was needed. The battery cell by Georges Leclanche proved out to be extremely advantageous in the early years of the telephones.
Dry Cell Battery
Metals used in a battery
About Author
Related Posts

Guidelines for Safer Micromobility Devices

Less Minerals for Batteries in Future

Consumer Standards for Lithium-Ion Batteries
Comments are closed.

IMAGES
COMMENTS
A 1919 illustration of a Leclanché cell. The Leclanché cell is a battery invented and patented by the French scientist Georges Leclanché in 1866. [1] [2] [3] The battery contained a conducting solution (electrolyte) of ammonium chloride, a cathode (positive terminal) of carbon, a depolarizer of manganese dioxide (oxidizer), and an anode (negative terminal) of zinc (reductant).
Feb 11, 2024 · Leclanche Cell is a zinc-carbon battery known as a dry cell and is widely used in devices such as flashlights and portable zinc-manganese dioxide systems. It was initially used in telegraphy, signaling, and electric bell work. In this article, we will see what Leclanche Cell is, its history, construction, applications, etc.
Jan 9, 2023 · Actually, it contained those same familiar elements of cathode, anode, electrolyte, terminals and case. Let’s take it apart in our minds, and figure out what once happened inside. The Secret Life of a Ground Breaking Leclanche Cell. The revolutionary battery cell George Leclanche cobbled together in 1865 was an unglazed clay pot.
May 14, 2020 · Construction. Leclanche Cell consists of a glass vessel containing a saturated solution of ammonium chloride (NH 4 Cl) as shown in figure 1. An amalgamated zinc rod (impure zinc rod covered with a layer of mercury) and a porous pot containing a carbon rod packed in a mixture of manganese dioxide (MnO 2) and powered coke are placed in the solution of NH 4 Cl.
The e.m.f. of a Leclanche cell is about 1.5 volts buts its resistance may amount to several ohms when a porous pot is employed. It was used extensively for telegraphy, signalling and electric bell work; and for most work where intermittent current is required and where it is essential that the battery should require very little attention from ...
Jun 4, 2023 · History of Leclanche Cell. The Leclanche cell is a type of battery that was invented in the 19th century. It was created by Georges Leclanche, a French engineer, in 1866. The cell consists of a zinc electrode (negative terminal), a carbon electrode (positive terminal), and a mixture of manganese dioxide and carbon powder as the cathode.
Objective: To verify the laws of combination (series) of resistances by using a meter bridge. Required Apparatus: A metre bridge, a Leclanche cell (battery eliminator), a galvanometer, a resistance box, a jockey, two resistance wires or two resistance coils of known resistances, a set square, connecting wires, a piece of sandpaper.
Das Leclanché-Element ist ein historisches galvanisches Element, das von Georges Leclanché entwickelt und 1866 patentiert wurde. Es stellt eine elektrische Batterie (Primärelement) dar und war in der ursprünglichen Form mit flüssigem Elektrolyt ausgestattet. Es zählt damit zu den heute nicht mehr verwendeten „Nassbatterien“.
Dec 11, 2014 · Going back to its history, the Leclanche cell was invented by the French engineer Georges Leclanche in 1866. Leclanche’s battery, additionally called a zinc-carbon battery, made use of an alternate type of cell than its antecedents. Rather than lead, the French engineer utilized zinc and a carbon-manganese dioxide mixture for his terminals.
Feb 7, 2023 · Leclanché Cells are the carbon-zinc primary batteries which have been largely replaced by alkaline cells. Leclanché Cells (zinc carbon or dry cell)