
- Why Does Water Expand When It Freezes
Gold Foil Experiment
- Faraday Cage
- Oil Drop Experiment
- Magnetic Monopole
- Why Do Fireflies Light Up
- Types of Blood Cells With Their Structure, and Functions
- The Main Parts of a Plant With Their Functions
- Parts of a Flower With Their Structure and Functions
- Parts of a Leaf With Their Structure and Functions
- Why Does Ice Float on Water
- Why Does Oil Float on Water
- How Do Clouds Form
- What Causes Lightning
- How are Diamonds Made
- Types of Meteorites
- Types of Volcanoes
- Types of Rocks

Who did the Gold Foil Experiment?
The gold foil experiment was a pathbreaking work conducted by scientists Hans Geiger and Ernest Marsden under the supervision of Nobel laureate physicist Ernest Rutherford that led to the discovery of the proper structure of an atom . Known as the Geiger-Marsden experiment, it was performed at the Physical Laboratories of the University of Manchester between 1908 and 1913.
The prevalent atomic theory at the time of the research was the plum pudding model that was developed by Lord Kelvin and further improved by J.J. Thomson. According to the theory, an atom was a positively charged sphere with the electrons embedded in it like plums in a Christmas pudding.
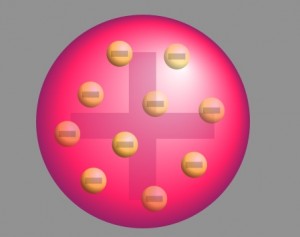
With neutrons and protons yet to be discovered, the theory was derived following the classical Newtonian Physics. However, in the absence of experimental proof, this approach lacked proper acceptance by the scientific community.
What is the Gold Foil Experiment?
Description.
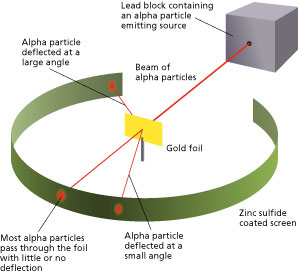
The method used by scientists included the following experimental steps and procedure. They bombarded a thin gold foil of thickness approximately 8.6 x 10 -6 cm with a beam of alpha particles in a vacuum. Alpha particles are positively charged particles with a mass of about four times that of a hydrogen atom and are found in radioactive natural substances. They used gold since it is highly malleable, producing sheets that can be only a few atoms thick, thereby ensuring smooth passage of the alpha particles. A circular screen coated with zinc sulfide surrounded the foil. Since the positively charged alpha particles possess mass and move very fast, it was hypothesized that they would penetrate the thin gold foil and land themselves on the screen, producing fluorescence in the part they struck.
Like the plum pudding model, since the positive charge of atoms was evenly distributed and too small as compared to that of the alpha particles, the deflection of the particulate matter was predicted to be less than a small fraction of a degree.
Observation
Though most of the alpha particles behaved as expected, there was a noticeable fraction of particles that got scattered by angles greater than 90 degrees. There were about 1 in every 2000 particles that got scattered by a full 180 degree, i.e., they retraced their path after hitting the gold foil.
Simulation of Rutherford’s Gold Foil Experiment Courtesy: University of Colorado Boulder
The unexpected outcome could have only one explanation – a highly concentrated positive charge at the center of an atom that caused an electrostatic repulsion of the particles strong enough to bounce them back to their source. The particles that got deflected by huge angles passed close to the said concentrated mass. Most of the particles moved undeviated as there was no obstruction to their path, proving that the majority of an atom is empty.
In addition to the above, Rutherford concluded that since the central core could deflect the dense alpha particles, it shows that almost the entire mass of the atom is concentrated there. Rutherford named it the “nucleus” after experimenting with various gases. He also used materials other than gold for the foil, though the gold foil version gained the most popularity.
He further went on to reject the plum pudding model and developed a new atomic structure called the planetary model. In this model, a vastly empty atom holds a tiny nucleus at the center surrounded by a cloud of electrons. As a result of his gold foil experiment, Rutherford’s atomic theory holds good even today.
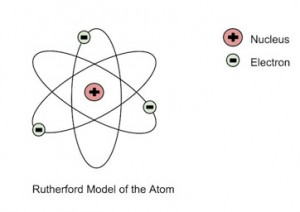
Rutherford’s Atomic Model
Rutherford’s Gold Foil Experiment Animation
- Rutherford demonstrated his experiment on bombarding thin gold foil with alpha particles contributed immensely to the atomic theory by proposing his nuclear atomic model.
- The nuclear model of the atom consists of a small and dense positively charged interior surrounded by a cloud of electrons.
- The significance and purpose of the gold foil experiment are still prevalent today. The discovery of the nucleus paved the way for further research, unraveling a list of unknown fundamental particles.
- Chemed.chem.purdue.edu
- Chem.libretexts.org
- Large.stanford.edu
- Radioa ctivity.eu.com
Article was last reviewed on Friday, February 3, 2023
Related articles

5 responses to “Gold Foil Experiment”
Super very much helpful to me,clear explanation about every act done by our Rutherford that is under different sub headings ,which is very much clear to ,to study .very much thanks to the science facts.com.thank u so much.
Good explanation,very helpful ,thank u ,so much
very clear and helpful, perfect for my science project!
Thank you for sharing the interactive program on the effects of the type of atom on the experiment! Looking forward to sharing this with my ninth graders!
Rutherford spearheaded with a team of scientist in his experiment of gold foil to capture the particles of the year 1911. It’s the beginning of explaining particles that float and are compacted . Rutherford discovered this atom through countless experiments which was the revolutionary discovery of the atomic nuclear . Rutherford name the atom as a positive charge and the the center is the nucleus.
Barack Hussein Obama
Mrs. Danize Obama
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Popular Articles

Join our Newsletter
Fill your E-mail Address
Related Worksheets
- Privacy Policy
© 2024 ( Science Facts ). All rights reserved. Reproduction in whole or in part without permission is prohibited.
About Rutherford's Gold Foil Experiment

Ernest Rutherford, originally from New Zealand, is credited as being the father of nuclear physics for his discoveries in atomic structure, even though Hantaro Nagaoka, a physicist from the Imperial University of Tokyo, first proposed the theory of the nucleus as it is known today. Rutherford's "gold foil experiment" led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. Prior to the groundbreaking gold foil experiment, Rutherford was granted the Nobel Prize for other key contributions in the field of chemistry.
The popular theory of atomic structure at the time of Rutherford's experiment was the "plum pudding model." This model was developed in 1904 by J.J. Thompson, the scientist who discovered the electron. This theory held that the negatively charged electrons in an atom were floating in a sea of positive charge–the electrons being akin to plums in a bowl of pudding. Although Dr. Nagaoka had published his competing theory that electrons orbit a positive nucleus, akin to the way the planet Saturn is orbited by its rings, in 1904, the plum pudding model was the prevailing theory on the structure of the atom until it was disproved by Ernest Rutherford in 1911.
The gold foil experiment was conducted under the supervision of Rutherford at the University of Manchester in 1909 by scientist Hans Geiger (whose work eventually led to the development of the Geiger counter) and undergraduate student Ernest Marsden. Rutherford, chair of the Manchester physics department at the time of the experiment, is given primary credit for the experiment, as the theories that resulted are primarily his work. Rutherford's gold foil experiment is also sometimes referred to as the Geiger-Marsden experiment.
The gold foil experiment consisted of a series of tests in which a positively charged helium particle was shot at a very thin layer of gold foil. The expected result was that the positive particles would be moved just a few degrees from their path as they passed through the sea of positive charge proposed in the plum pudding model. The result, however, was that the positive particles were repelled off of the gold foil by nearly 180 degrees in a very small region of the atom, while most of the remaining particles were not deflected at all but rather passed right through the atom.
Significance
The data generated from the gold foil experiment demonstrated that the plum pudding model of the atom was incorrect. The way in which the positive particles bounced off the thin foil indicated that the majority of the mass of an atom was concentrated in one small region. Because the majority of the positive particles continued on their original path unmoved, Rutherford correctly deducted that most of the remainder of the atom was empty space. Rutherford termed his discovery "the central charge," a region later named the nucleus.
Rutherford's discovery of the nucleus and proposed atomic structure was later refined by physicist Niels Bohr in 1913. Bohr's model of the atom, also referred to as the Rutherford Bohr model, is the basic atomic model used today. Rutherford's description of the atom set the foundation for all future atomic models and the development of nuclear physics.
Cite This Article
Pestka, Jessica. "About Rutherford's Gold Foil Experiment" sciencing.com , https://www.sciencing.com/rutherfords-gold-foil-experiment-4569065/. 24 April 2017.
Pestka, Jessica. (2017, April 24). About Rutherford's Gold Foil Experiment. sciencing.com . Retrieved from https://www.sciencing.com/rutherfords-gold-foil-experiment-4569065/
Pestka, Jessica. About Rutherford's Gold Foil Experiment last modified March 24, 2022. https://www.sciencing.com/rutherfords-gold-foil-experiment-4569065/
Recommended
What is the 'Gold Foil Experiment'? The Geiger-Marsden experiments explained
Physicists got their first look at the structure of the atomic nucleus.

J.J. Thomson model of the atom
Gold foil experiments, rutherford model of the atom.
- The real atomic model
Additional Resources
Bibliography.
The Geiger-Marsden experiment, also called the gold foil experiment or the α-particle scattering experiments, refers to a series of early-20th-century experiments that gave physicists their first view of the structure of the atomic nucleus and the physics underlying the everyday world. It was first proposed by Nobel Prize -winning physicist Ernest Rutherford.
As familiar as terms like electron, proton and neutron are to us now, in the early 1900s, scientists had very little concept of the fundamental particles that made up atoms .
In fact, until 1897, scientists believed that atoms had no internal structure and believed that they were an indivisible unit of matter. Even the label "atom" gives this impression, given that it's derived from the Greek word "atomos," meaning "indivisible."

But that year, University of Cambridge physicist Joseph John Thomson discovered the electron and disproved the concept of the atom being unsplittable, according to Britannica . Thomson found that metals emitted negatively charged particles when illuminated with high-frequency light.
His discovery of electrons also suggested that there were more elements to atomic structure. That's because matter is usually electrically neutral; so if atoms contain negatively charged particles, they must also contain a source of equivalent positive charge to balance out the negative charge.
By 1904, Thomson had suggested a "plum pudding model" of the atom in which an atom comprises a number of negatively charged electrons in a sphere of uniform positive charge, distributed like blueberries in a muffin.
The model had serious shortcomings, however — primarily the mysterious nature of this positively charged sphere. One scientist who was skeptical of this model of atoms was Rutherford, who won the Nobel Prize in chemistry for his 1899 discovery of a form of radioactive decay via α-particles — two protons and two neutrons bound together and identical to a helium -4 nucleus, even if the researchers of the time didn't know this.
Rutherford's Nobel-winning discovery of α particles formed the basis of the gold foil experiment, which cast doubt on the plum pudding model. His experiment would probe atomic structure with high-velocity α-particles emitted by a radioactive source. He initially handed off his investigation to two of his protégés, Ernest Marsden and Hans Geiger, according to Britannica .
Rutherford reasoned that if Thomson's plum pudding model was correct, then when an α-particle hit a thin foil of gold, the particle should pass through with only the tiniest of deflections. This is because α-particles are 7,000 times more massive than the electrons that presumably made up the interior of the atom.
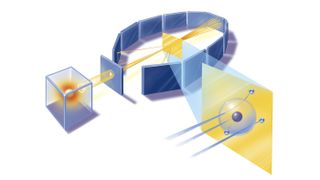
Marsden and Geiger conducted the experiments primarily at the Physical Laboratories of the University of Manchester in the U.K. between 1908 and 1913.
The duo used a radioactive source of α-particles facing a thin sheet of gold or platinum surrounded by fluorescent screens that glowed when struck by the deflected particles, thus allowing the scientists to measure the angle of deflection.
The research team calculated that if Thomson's model was correct, the maximum deflection should occur when the α-particle grazed an atom it encountered and thus experienced the maximum transverse electrostatic force. Even in this case, the plum pudding model predicted a maximum deflection angle of just 0.06 degrees.
Of course, an α-particle passing through an extremely thin gold foil would still encounter about 1,000 atoms, and thus its deflections would be essentially random. Even with this random scattering, the maximum angle of refraction if Thomson's model was correct would be just over half a degree. The chance of an α-particle being reflected back was just 1 in 10^1,000 (1 followed by a thousand zeroes).
Yet, when Geiger and Marsden conducted their eponymous experiment, they found that in about 2% of cases, the α-particle underwent large deflections. Even more shocking, around 1 in 10,000 α-particles were reflected directly back from the gold foil.
Rutherford explained just how extraordinary this result was, likening it to firing a 15-inch (38 centimeters) shell (projectile) at a sheet of tissue paper and having it bounce back at you, according to Britannica
Extraordinary though they were, the results of the Geiger-Marsden experiments did not immediately cause a sensation in the physics community. Initially, the data were unnoticed or even ignored, according to the book "Quantum Physics: An Introduction" by J. Manners.
The results did have a profound effect on Rutherford, however, who in 1910 set about determining a model of atomic structure that would supersede Thomson's plum pudding model, Manners wrote in his book.
The Rutherford model of the atom, put forward in 1911, proposed a nucleus, where the majority of the particle's mass was concentrated, according to Britannica . Surrounding this tiny central core were electrons, and the distance at which they orbited determined the size of the atom. The model suggested that most of the atom was empty space.
When the α-particle approaches within 10^-13 meters of the compact nucleus of Rutherford's atomic model, it experiences a repulsive force around a million times more powerful than it would experience in the plum pudding model. This explains the large-angle scatterings seen in the Geiger-Marsden experiments.
Later Geiger-Marsden experiments were also instrumental; the 1913 tests helped determine the upper limits of the size of an atomic nucleus. These experiments revealed that the angle of scattering of the α-particle was proportional to the square of the charge of the atomic nucleus, or Z, according to the book "Quantum Physics of Matter," published in 2000 and edited by Alan Durrant.
In 1920, James Chadwick used a similar experimental setup to determine the Z value for a number of metals. The British physicist went on to discover the neutron in 1932, delineating it as a separate particle from the proton, the American Physical Society said .
What did the Rutherford model get right and wrong?
Yet the Rutherford model shared a critical problem with the earlier plum pudding model of the atom: The orbiting electrons in both models should be continuously emitting electromagnetic energy, which would cause them to lose energy and eventually spiral into the nucleus. In fact, the electrons in Rutherford's model should have lasted less than 10^-5 seconds.
Another problem presented by Rutherford's model is that it doesn't account for the sizes of atoms.
Despite these failings, the Rutherford model derived from the Geiger-Marsden experiments would become the inspiration for Niels Bohr 's atomic model of hydrogen , for which he won a Nobel Prize in Physics .
Bohr united Rutherford's atomic model with the quantum theories of Max Planck to determine that electrons in an atom can only take discrete energy values, thereby explaining why they remain stable around a nucleus unless emitting or absorbing a photon, or light particle.
Thus, the work of Rutherford, Geiger (who later became famous for his invention of a radiation detector) and Marsden helped to form the foundations of both quantum mechanics and particle physics.
Rutherford's idea of firing a beam at a target was adapted to particle accelerators during the 20th century. Perhaps the ultimate example of this type of experiment is the Large Hadron Collider near Geneva, which accelerates beams of particles to near light speed and slams them together.
- See a modern reconstruction of the Geiger-Marsden gold foil experiment conducted by BackstageScience and explained by particle physicist Bruce Kennedy .
- Find out more about the Bohr model of the atom which would eventually replace the Rutherford atomic model.
- Rutherford's protege Hans Gieger would eventually become famous for the invention of a radioactive detector, the Gieger counter. SciShow explains how they work .
Thomson's Atomic Model , Lumens Chemistry for Non-Majors,.
Rutherford Model, Britannica, https://www.britannica.com/science/Rutherford-model
Alpha particle, U.S NRC, https://www.nrc.gov/reading-rm/basic-ref/glossary/alpha-particle.html
Manners. J., et al, 'Quantum Physics: An Introduction,' Open University, 2008.
Durrant, A., et al, 'Quantum Physics of Matter,' Open University, 2008
Ernest Rutherford, Britannica , https://www.britannica.com/biography/Ernest-Rutherford
Niels Bohr, The Nobel Prize, https://www.nobelprize.org/prizes/physics/1922/bohr/facts/
House. J. E., 'Origins of Quantum Theory,' Fundamentals of Quantum Mechanics (Third Edition) , 2018
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Robert Lea is a science journalist in the U.K. who specializes in science, space, physics, astronomy, astrophysics, cosmology, quantum mechanics and technology. Rob's articles have been published in Physics World, New Scientist, Astronomy Magazine, All About Space and ZME Science. He also writes about science communication for Elsevier and the European Journal of Physics. Rob holds a bachelor of science degree in physics and astronomy from the U.K.’s Open University
Large Hadron Collider finds 1st evidence of the heaviest antimatter particle yet
Do atoms ever touch?
NASA's Parker Solar Probe will reach its closest-ever point to the sun on Christmas Eve
Most Popular
- 2 Everything you need to know about digiscoping
- 3 MIT's massive database of 8,000 new AI-generated EV designs could shape how the future of cars look
- 4 'Rising temperatures melted corpses out of the Antarctic permafrost': The rise of one of Earth's most iconic trees in an uncertain world
- 5 Space photo of the week: James Webb and Chandra spot a cosmic 'Christmas Wreath' sparkling in the galaxy next door

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons

Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

4.14: Gold Foil Experiment
- Last updated
- Save as PDF
- Page ID 52928
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
How much space do bricks occupy?
As we look at the world around us, it looks pretty solid. We hit a wall with our hand and the hand stops – it does not (normally) go through the wall. We think of matter as occupying space. But there is a lot of empty space in matter. In fact, most of the matter is empty space.
The Gold Foil Experiment
In 1911, Rutherford and coworkers Hans Geiger and Ernest Marsden initiated a series of groundbreaking experiments that would completely change the accepted model of the atom. They bombarded very thin sheets of gold foil with fast moving alpha particles . Alpha particles, a type of natural radioactive particle, are positively charged particles with a mass about four times that of a hydrogen atom.
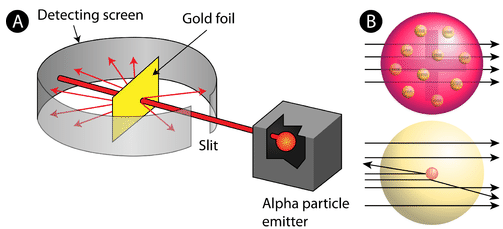
According to the accepted atomic model—in which an atom's mass and charge are uniformly distributed throughout the atom—the scientists hypothesized that all of the alpha particles would pass through the gold foil with only a slight deflection, or none at all. Surprisingly, while most of the alpha particles were indeed not deflected, a very small percentage (about 1 in 8000 particles) bounced off the gold foil at very large angles. Some were even redirected back toward the source. No prior knowledge had prepared them for this discovery. In a famous quote, Rutherford exclaimed that it was "as if you had fired a 15-inch [artillery] shell at a piece of tissue and it came back and hit you."
Rutherford needed to come up with an entirely new model of the atom in order to explain his results. Because the vast majority of the alpha particles had passed through the gold, he reasoned that most of the atom was empty space. In contrast, the particles that were highly deflected must have experienced a tremendously powerful force within the atom. He concluded that all of the positive charge and the majority of the mass of the atom must be concentrated in a very small space in the atom's interior, which he called the nucleus. The nucleus is the tiny, dense, central core of the atom and is composed of protons and neutrons.
Rutherford's atomic model became known as the nuclear model . In the nuclear atom, the protons and neutrons, which comprise nearly all of the mass of the atom, are located in the nucleus at the center of the atom. The electrons are distributed around the nucleus and occupy most of the volume of the atom. It is worth emphasizing just how small the nucleus is compared to the rest of the atom. If we could blow up an atom to be the size of a large professional football stadium, the nucleus would be about the size of a marble.
Rutherford's model proved to be an important step towards a full understanding of the atom. However, it did not completely address the nature of the electrons and the way in which they occupied the vast space around the nucleus. It was not until some years later that a full understanding of the electron was achieved. This proved to be the key to understanding the chemical properties of elements.
- Bombardment of gold foil with alpha particles showed that a very small percentage of alpha particles were deflected.
- The nuclear model of the atom consists of a small and dense positively charged interior surrounded by a cloud of electrons.
- What is an alpha particle?
- What did Rutherford observe from shooting thousands and thousands of alpha particles at a thin piece of gold foil?
- How did Rutherford explain the observation that most alpha particles went straight through the gold foil?
- What did he say about the particles that were deflected?
- Describe Rutherford’s nuclear model.

Discovering the Nucleus: Rutherford’s Gold Foil Experiment

History of Chemistry: Rutherford Gold Foil Experiment
In this article, you will learn the history behind the Rutherford Gold Foil Experiment and the events that led to the discovery of the atomic nucleus. If you enjoy this article, check out our other history of chemistry articles linked below!
- Rutherford Atomic Model
- JJ Thompson cathode-ray tube
- Rutherfords Jar Experiment
- Molecular Geometry tutorial
- The structure of an atom
- Bohr Atomic Model
- Nuclear Reactions
Who was Ernest Rutherford?

Ernest Rutherford is known as the father of nuclear physics. Born in Brightwater, New Zealand on August 30th, 1871, Rutherford was the fourth of twelve children. His father was a farmer and his mother a school teacher. From a very early age, Rutherford understood the importance of hard work and the power of education. In school, he excelled greatly and at the age of fifteen won an academic scholarship to study at Nelson Collegiate School. Then, at the age of 19, he won another academic scholarship to study at Canterbury College in Christchurch. A few years later he won another scholarship, the exhibition science scholarship, and he left New Zealand to study at Trinity College, Cambridge in England. While there, he conducted research at the Cavendish Laboratory under his advisor J.J. Thomson .

During his time at Cavendish Lab, Rutherford faced adversity from his peers. Because he was from New Zealand, he was often ostracized by fellow students. In the end, he used this as motivation to succeed. Which he did as he made a multitude of great discoveries through his research in gases and radioactivity. These included the discovery of different types of radiation, radiometric dating, and the nucleus of an atom.
The Rutherford Gold Foil Experiment
The experiment.
While working as a chair at the University of Manchester, Rutherford conducted the gold-foil experiment alongside Hans Geiger and Ernest Marsden. In this experiment, they shot alpha particles –which Rutherford had discovered years prior– directly at a piece of thin gold foil . As the alpha particles passed through, they would hit the phosphorescent screen encasing the foil. When the particles came into contact with the screen, there would be a flash.

Observations
Going into the experiment, Rutherford had formed preconceptions for the experiment based on J.J. Thomson’s plum pudding model . He predicted the alpha particles would shoot through the foil with ease. Some of the particles did manage to pass directly through the foil, but some veered from the path either bouncing back or deflecting. Rutherford found this to be an exciting observation and compared it to shooting a bullet at a piece of tissue and having it bounce back.
From this observation, two deductions were made. Firstly, he concluded most of the atom is composed of empty space. Secondly, he concluded there must be something small, dense, and positive inside the atom to repel the positively charged alpha particles. This became the nucleus, which in Latin means the seed inside of a fruit.
The Nuclear Model
The gold-foil experiment disproved J.J. Thomsons plum pudding model, which hypothesized the atom was positively charged spaced with electrons embedded inside. Therefore, giving way to the nuclear model. In this model, Rutherford theorized the atomic structure was similar to that of the solar system. Where the nucleus was in this middle and surrounded by empty space with orbiting electrons.

Home » Science » Physics » What is Rutherford’s Gold Foil Experiment
What is Rutherford’s Gold Foil Experiment
Rutherford’s gold foil experiment (Rutherford’s alpha particle scattering experiment) refers to an experiment carried out by Ernest Rutherford, Hans Geiger, and Ernest Marsden at the University of Manchester in the early 1900s. In the experiment, Rutherford and his two students studied how alpha particles fired at a thin piece of gold foil were deflected. According to the popular atomic models of the time, all of the alpha particles should have traveled straight through the gold foil. However, to their surprise, Rutherford and his students found that around 1 in every 8000 alpha particles were deflected back towards the source (i.e. at angles larger than 90 o ). In order to explain this effect, they had to come up with a new model (now known as the “ Rutherford Model “) for the atom.

Ernest Rutherford
For the experiment, a radioactive source which emits alpha particles was kept in front of a thin gold foil. The source and the gold foil was surrounded by a screen with a zinc sulphide coating, and the air was pumped out to ensure that the equipment were all within a vacuum. (If they had not, the alpha particles would have used up their energy to ionise air molecules and may have never reached the gold foil).
The alpha particles emitted by the source were expected to pass straight through the gold foil. Whenever they hit the zinc sulphide coated screen, they were to produce a small glowing spot on the screen.
The popular model for the atom at the time was known as the “ Plum Pudding Model “. This was a model developed by J.J. Thomson, who had discovered electrons a few years earlier. According to his model, atoms were spherical objects, with the positive charge evenly spread throughout like a dough, and little bits of negative charge (electrons) sticking on it like plums. If this “Plum Pudding Model” had been correct, all of the alpha particles should have passed straight through the gold atoms in the gold foil, showing very little deflection. However, what Rutherford and his students observed was quite different.
Most of the alpha particles did go straight through the gold foil. However, a few of the alpha particles seemed to be deflected at large angles. Rarely, some alpha particles even seemed to have been deflected by angles larger than 90 0 . To explain this result, Rutherford proposed that the mass of an atom must be concentrated in a very small area at the centre, which he called the “nucleus”. From the deflections, it was also clear that the nucleus was charged:

Rutherford’s Gold Foil Experiment – Geiger-Marsden experiment expectation and result
Rutherford’s Gold Foil Experiment – Main Observations and Conclusions
Rutherford did not necessarily determine that the nucleus was positively charged during these early experiments (the deflections could have been produced by attractive negative charges rather than repulsive positive charges at the centre). Rutherford eventually did discover that the nucleus of an atom was positively charged, but this was done in a different experiment.
Eventually, Niels Bohr and Erwin Schrödinger came up with better models for atoms, but Rutherford’s gold foil experiment remains one of the most groundbreaking experiments in the history of physics.
Image Courtesy:
1. “ernest rutherford 1892” by unknown, published in 1939 in rutherford: being the life and letters of the rt. hon. lord rutherford, o. m [ cc by 4.0 ], via wikimedia commons, 2. “geiger-marsden experiment expectation and result” by kurzon (own work) [ cc by-sa 3.0 ], via wikimedia commons.
About the Author: Nipun
you may also like these.
The Gold Foil Experiment: Unveiling the Nuclear Atom
Expert reviewed • 22 November 2024 • 6 minute read
Introduction
The Geiger-Marsden gold foil experiment, conducted in 1909, ranks among the most significant experiments in atomic physics. This elegant investigation led Ernest Rutherford to propose the nuclear model of the atom, fundamentally changing our understanding of atomic structure.
The Experimental Setup
The experiment used the following components:
- A radioactive source producing alpha particles (helium nuclei)
- A thin gold foil approximately 100 atoms thick
- A fluorescent screen to detect scattered alpha particles
- A microscope to observe scintillations on the screen
Key Observations and Findings
The experiment revealed two crucial observations:
Primary Observation : Most alpha particles passed straight through the gold foil without deflection, suggesting that atoms were mostly empty space.
Critical Discovery : A small fraction of alpha particles (approximately 1 in 8000) scattered at large angles, with some even bouncing directly backward.
The mathematical relationship describing the scattering of alpha particles is given by Rutherford's scattering formula:
- d N d Ω \frac{dN}{d\Omega} d Ω d N is the differential scattering cross-section
- Z 1 Z_1 Z 1 and Z 2 Z_2 Z 2 are the atomic numbers
- e e e is the elementary charge
- ϵ 0 \epsilon_0 ϵ 0 is the vacuum permittivity
- m m m is the mass of the alpha particle
- v v v is the velocity of the alpha particle
- θ \theta θ is the scattering angle
Rutherford's Nuclear Model
Based on these observations, Rutherford proposed a new model of the atom with these key features:
- A dense, positively charged nucleus containing most of the atom's mass
- Electrons orbiting the nucleus like planets around the sun
- Mostly empty space between the nucleus and electrons
Discovery of the Proton
Following the gold foil experiment, Rutherford conducted further investigations that led to the discovery of the proton in 1919. When alpha particles bombarded nitrogen gas, the following nuclear reaction occurred:
Limitations of the Nuclear Model
Despite its groundbreaking nature, Rutherford's model had three significant limitations:
- Orbital Stability : According to classical electromagnetic theory, orbiting electrons should emit radiation and spiral into the nucleus. The mathematical expression for the power radiated by an accelerating charge is:
- P P P is the radiated power
- a a a is the acceleration
- c c c is the speed of light
Electron Behavior : The model couldn't explain electron energy levels or atomic spectra.
Nuclear Composition : The model couldn't fully account for atomic mass, later resolved by Chadwick's discovery of the neutron.
Millikan's Oil Drop Experiment
The Discovery of the Neutron: Chadwick's Groundbreaking Experiment
Return to Module *: From the Universe to the Atom

IMAGES
COMMENTS
Feb 3, 2023 · The gold foil experiment was a pathbreaking work conducted by scientists Hans Geiger and Ernest Marsden under the supervision of Nobel laureate physicist Ernest Rutherford that led to the discovery of the proper structure of an atom. Known as the Geiger-Marsden experiment, it was performed at the Physical Laboratories of the University of ...
Apr 24, 2017 · Rutherford's "gold foil experiment" led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. Ernest Rutherford, originally from New Zealand, is credited as being the father of nuclear physics for his discoveries in atomic structure, even though Hantaro Nagaoka, a physicist from the Imperial ...
The atomic weight of gold was known to be around 197 since early in the 19th century. [65] From an experiment in 1906, Rutherford measured alpha particles to have a charge of 2 q e and an atomic weight of 4, and alpha particles emitted by radon to have velocity of 1.70 × 10 7 m/s. [66]
Feb 12, 2022 · The Geiger-Marsden experiment, also called the gold foil experiment or the α-particle scattering experiments, refers to a series of early-20th-century experiments that gave physicists their first ...
Sep 20, 2022 · The Gold Foil Experiment. In 1911, Rutherford and coworkers Hans Geiger and Ernest Marsden initiated a series of groundbreaking experiments that would completely change the accepted model of the atom. They bombarded very thin sheets of gold foil with fast moving alpha particles. Alpha particles, a type of natural radioactive particle, are ...
A piece of gold foil was hit with alpha particles, which have a positive charge. Most alpha particles went right through. This showed that the gold atoms were mostly empty space. Some particles had their paths bent at large angles. A few even bounced backward.
The Rutherford Gold Foil Experiment The Experiment. While working as a chair at the University of Manchester, Rutherford conducted the gold-foil experiment alongside Hans Geiger and Ernest Marsden. In this experiment, they shot alpha particles–which Rutherford had discovered years prior– directly at a piece of thin gold foil. As the alpha ...
Jul 28, 2015 · For the experiment, a radioactive source which emits alpha particles was kept in front of a thin gold foil. The source and the gold foil was surrounded by a screen with a zinc sulphide coating, and the air was pumped out to ensure that the equipment were all within a vacuum. (If they had not, the alpha particles would have used up their energy ...
Nov 22, 2024 · The Geiger-Marsden gold foil experiment, conducted in 1909, ranks among the most significant experiments in atomic physics. This elegant investigation led Ernest Rutherford to propose the nuclear model of the atom, fundamentally changing our understanding of atomic structure. The Experimental Setup. The experiment used the following components:
Rutherford’s Gold Foil Experiment In 1909, Rutherford enlisted his assistant, Hans Geiger, and a research student, Ernest Marsden to investigate the scattering of alpha particles, positively charged particles, upon different metals. In the experiment a glass conical tube was filled with radium emanation and sealed at the cone with a thin