- High School
- You don't have any recent items yet.
- You don't have any courses yet.
- You don't have any books yet.
- You don't have any Studylists yet.
- Information

Amylase Lab Report
Human anatomy and physiology ii (bio 2312), new york city college of technology, students also viewed.
- Lecture 12 Endocrine System
- Urinalysis Lab report
- Excercse 33 part 2 - A&P2 Excercise 33 Part 2 Homework
- Review Questions A&P II - Blood
- Urinary system - Urine analysis review questions and answers
- BIO2312 - prof s
Related documents
- LAB practice question
- BIO2312 Circulation Of Blood Lecture
- BIO2312 LEC THE Endocrine System
- A and P II Lab Practicum List of Organs and Structures
- Cardiovascular Blood Circulatory System Heart
Related Studylists
Preview text.
Susanna Conigliaro Biology 2312 Lab Report Title: Effects of Amylase on Starch Breakdown Introduction Amylase is an enzyme who's primary function is to break down starch into simpler sugars. In the lab an experiment was performed to determine the effect of amylase in different situations. To determine the effect of amylase tests using iodine and Benedict's test were used. The Benedict’s test uses Benedict’s reagent which is used to detect the presence of simple carbohydrates ( “Organic Molecules,” n.). The Benedicts’s test is a qualitative test since it detects the presence of simple carbohydrates with a change in color. The iodine test is another qualitative test which changes from an orange to a dark blue color when in the presence of starch. The iodine changes colors since it adheres to amylose molecules and reflects back a dark color (Byrid-McDevitt, 2016). The two tests are great indicators to test the effect of amylase. Amylase is an enzyme which breaks down starch into simple sugars. Digestion is the process of breaking down food through the use of mechanical and chemical processes. Amylase is an enzyme which is naturally found in saliva and is responsible for the chemical digestion that occurs in the mouth. When someone eats a piece of bread the salivary amylase in the mouth coats the bread and starts to break down the carbohydrate into simpler sugars. In the lab amylase was used in different tests to show the effectiveness of the enzyme.
Objective: The objective for the lab is to examine the effect of amylase on starch.
Methods and Materials To carry out the experiment materials the necessary materials were collected and organized. First a beaker of water was set to boil, and while waiting six tubes were collected and labeled to carry out six different tests. In test tube #1 6 gtts of amylase and water were added. In test tube #2 6 gtts of starch and water were added. In test tube #3 6 gtts of maltose and water were added. In test tube #4 6 gtts of amylase was added and then out to boil for four minutes before adding 6 gtts of starch. In test tube #5 6 gtts of amylase and starch were added. In test tube #6 6 gtts of amylase and starch were added. Once the test tubes were set up, the tubes were then transferred to their proper incubating places. Test tubes #1- #5 were placed in an incubator at 37 degrees Celsius while test tube #6 was placed in an ice tub for 20 minutes. After the time elapsed a pipette was used to transfer a sample from each tube onto paper to perform the iodine test. Each sample was labeled to avoid confusion and then a drop of iodine was added to each. The results from the test were then recorded. The test tubes were then used to perform the Benedict’s reagent test. A drop of Benedict’s reagent was added to each test tube and then placed in boiling water for 5 minutes. After 5 minutes the tubes were removed from the water and the data was collected regarding the change in each test tube’s color.
Picture 1. The picture to the left shows the different solution that were used for the tests.
Picture 2 . Pictured to the left is the Iodine and Benedict’s reagent which was used to perform the two tests.
Picture 3. The picture to the left shows the results for the Iodine test. According to the results test tubes #2 and #4 are positive for the presence of starch while test tubes # 1,3, 5, and 6 are negative.
Picture 4. The picture to the left shows the test tubes prior to being boiled for the Benedict’s reagents test.
Picture 5. The picture to the left shows the results of the Bendict’s reagents which shows that test tube #2 is negative and test tubes #1, 3, 4, 5, and 6 are positive for the presence of simple carbohydrates.
Discussion The six different tests results help to explain the effect of amylase on its substrate. Test tube #1 was a control test for the iodine test since it did not contain the substrate so no starch was present. However, looking at the results there may have been an issue with the test since the Benedict’s test was positive. This could be a result from human error in which the sample placed for the test could have been from a mistake and taken from test tube #5. Test tube #2 acted as a
References Byrid-McDevitt, L. (2016, September 23). Saturday Science: Incredible Iodine. Retrieved November 24, 2018, from childrensmuseum/blog/saturday-science- incredible-iodine
Organic Molecules. (n.). Retrieved November 24, 2018, from nku/
~whitsonma/bio150lsite/lab 3 organic/bio150lrevmolec.
- Multiple Choice
Course : Human Anatomy And Physiology II (BIO 2312)
University : new york city college of technology.

- Discover more from: Human Anatomy And Physiology II BIO 2312 New York City College of Technology 24 Documents Go to course
- More from: Human Anatomy And Physiology II BIO 2312 New York City College of Technology 24 Documents Go to course
- More from: API by Treeshaholic 20 20 documents Go to Studylist
Practical Biology
A collection of experiments that demonstrate biological concepts and processes.

Observing earthworm locomotion

Practical Work for Learning

Published experiments
Investigating the effect of ph on amylase activity, class practical.
Measure the time taken for amylase to completely break down starch , by withdrawing samples at 10 second intervals and noting the time at which the solution no longer gives a blue-black colour with iodine solution (but the iodine solution remains orange). Use buffers to provide solutions at different pHs . Calculate the rate of this enzyme controlled reaction by calculating 1÷ time.
Lesson organisation
This procedure is simple enough for individuals to carry out if you have enough dimple tiles. If you choose to investigate five pHs, then groups of five students could complete the investigation by working together and pooling results.

Apparatus and Chemicals
For each group of students:.
Syringes, 5 cm 3 , 2 (1 for starch, 1 for amylase)
Iodine solution in a dropper bottle ( Note 4 )
Test tube rack
Test tube, 1 for each pH to be tested
Dimple tile or white tiles
Teat pipette
For the class – set up by technician/ teacher:
Amylase 1% (or 0.5%) ( Note 1 )
Starch 1% (or 0.5%) ( Note 2 )
Buffer solutions covering a range of pH, each with a labelled syringe/ plastic pipette ( Note 3 )
Health & Safety and Technical notes
Amylase solution and iodine solution are low hazard once made up. Wear eye protection when handling iodine solution. Hazards of buffers may vary. See CLEAPSS Recipe card or supplier’s information and see Note 3 .
Read our standard health & safety guidance
1 Amylase (See CLEAPSS Hazcard and Recipe card) The powdered enzyme is HARMFUL, but solutions less than 1% are LOW HAZARD. It is wise to test, well in advance, the activity of the stored enzyme at its usual working concentration to check that substrates are broken down at an appropriate rate. Enzymes may degrade in storage and this allows time to adjust concentrations or to obtain fresh stocks. Amylase will slowly lose activity, so it is best to make up a fresh batch for each lesson; batches may vary in activity and results collected on different days will not be comparable. The optimum temperature for your enzyme will be listed on the supplier’s label.
Using saliva: the CLEAPSS Laboratory Handbook provides guidance on precautions to take (including hygiene precautions) in order to use saliva safely as a source of amylase. This has the advantage of being cheaper, not requiring technicians to make up fresh solutions each lesson, it is directly interesting to students, and salivary amylase is reliable. It also provides an opportunity to teach good hygiene precautions – including ensuring that students use only their own saliva samples (provide small beakers to spit into); that students are responsible for rinsing their own equipment; and that all contaminated glassware is placed in a bowl or bucket of sodium chlorate(I) before technicians wash up.
2 Starch suspension – make fresh. Make a cream of 5 g soluble starch in cold water. Pour into 500 cm 3 of boiling water and stir well. Boil until you have a clear solution. Do not use modified starch.
3 Buffers: (See CLEAPSS Recipe card) If you make universal buffer it will contain sodium hydroxide at approximately 0.25 M, and should be labelled IRRITANT. Refer to other relevant Hazcards if you choose to make other buffers, or to supplier’s information if you purchase buffer solutions/ tablets. ( Note 1 )
4 Iodine solution (See CLEAPSS Hazcard and Recipe card). A 0.01 M solution is suitable for starch testing. Make this by 10-fold dilution of 0.1 M solution. Once made, the solution is a low hazard but may stain skin or clothing if spilled.
Ethical issues
There are no ethical issues associated with this procedure.
SAFETY: All solutions once made up are low hazard. Wear eye protection, as iodine may irritate eyes.
Preparation
a Check the speed of the reaction with the suggested volumes of reactants to be used – 2 cm 3 of starch: 2 cm 3 of amylase: 1 cm 3 of buffer at pH 6. Ideally the reaction should take about 60 seconds at this pH: this is the usual optimum for amylase (see note 1). If the reaction is too fast, either reduce the enzyme volume or increase the starch volume. If the reaction is too slow, increase the enzyme volume or concentration or reduce the starch volume or concentration.
Investigation

b Place single drops of iodine solution in rows on the tile.
c Label a test tube with the pH to be tested.
d Use the syringe to place 2 cm 3 of amylase into the test tube.
e Add 1 cm 3 of buffer solution to the test tube using a syringe.
f Use another syringe to add 2 cm 3 of starch to the amylase/ buffer solution, start the stop clock and leave it on throughout the test. Mix using a plastic pipette.
g After 10 seconds, use the plastic pipette to place one drop of the mixture on the first drop of iodine. The iodine solution should turn blue-black. If the iodine solution remains orange the reaction is going too fast and the starch has already been broken down. Squirt the rest of the solution in the pipette back into the test tube.
h Wait another 10 seconds. Then remove a second drop of the mixture to add to the next drop of iodine.
i Repeat step h until the iodine solution and the amylase/ buffer/ starch mixture remain orange.
j You could prepare a control drop for comparison with the test drops. What should this contain?
k Count how many iodine drops you have used, each one equalling 10 seconds of reaction time.
l Repeat the whole procedure with another of the pH buffers to be used, or pool the class results.
m Consider collecting repeat data if there is time.
n Plot a graph of time taken to break down starch against pH, or calculate the rate of reaction and plot rate against pH.
Teaching notes
This is a straightforward practical giving reliable, unambiguous results. The main errors will be in the order of mixing the enzyme/ substrate/ buffer, or a delay in sampling so that the reaction time is under-estimated or rate is over-estimated. Temperature variation affects enzyme activity, so results collected on different days are not comparable.
Health and safety checked, September 2008
http://rsc.org/Education/Teachers/Resources/cfb/enzymes.htm Royal Society of Chemistry: Chemistry for Biologists: Enzymes
A clear and thorough presentation of information about enzymes as chemical catalysts and the factors affecting their activity.
(Website accessed October 2011)
- OCR Gateway
What happens in cells (and what do cells need)? - OCR Gateway The effect of pH on the reaction rate of amylase
The genetic code of all life on Earth is made from DNA. Proteins like enzymes and hormones are made during protein synthesis. Enzymes are biological catalysts which speed up chemical reactions.
Part of Biology (Single Science) Cell level systems
Save to My Bitesize
Practical - The effect of pH on the rate of reaction of amylase
To determine the rate of the amylase close amylase An enzyme that can break down starch into simple sugars. activity at different pHs close pH Scale of acidity or alkalinity. A pH (power of hydrogen) value below 7 is acidic, a pH value above 7 is alkaline. .
You will investigate the breakdown of starch close starch A type of carbohydrate. Plants can turn the glucose produced in photosynthesis into starch for storage, and turn it back into glucose when it is needed for respiration. by amylase at different pHs.
The different pHs under investigation will be produced using buffer solutions close buffer solution A solution that is used to produce a particular pH, and will maintain this pH if acids or alkalis are added. . Buffer solutions produce a particular pH, and will maintain it if other substances are added.
The amylase will break down the starch.
A series of test tubes containing a mixture of starch and amylase is set up at different pHs.
A sample is removed from the test tubes every 10 seconds to test for the presence of starch. Iodine solution close iodine solution A solution of iodine in potassium iodide solution, also referred to as potassium triiodide solution. will turn a blue/black colour when starch is present, so when all the starch is broken down, a blue-black colour is no longer produced. The iodine solution will remain orange-brown.
A control close control A part of the experiment in which all the variables except the dependent variable are kept the same. A control lets you observe the effect (if any) of changing the independent variable. experiment must be set up - without the amylase - to make sure that the starch would not break down anyway, in its absence. The result of the control experiment must be negative - the colour must remain blue-black - for results with the enzyme to be valid close valid experiment An experiment is valid when it does what it sets out to do. .
When the starch solution is added:
- start timing immediately
- remove a sample immediately, and test it with iodine solution
- sample the starch-amylase mixture continuously, for example every 10 seconds
This is how you might set up the experiment:
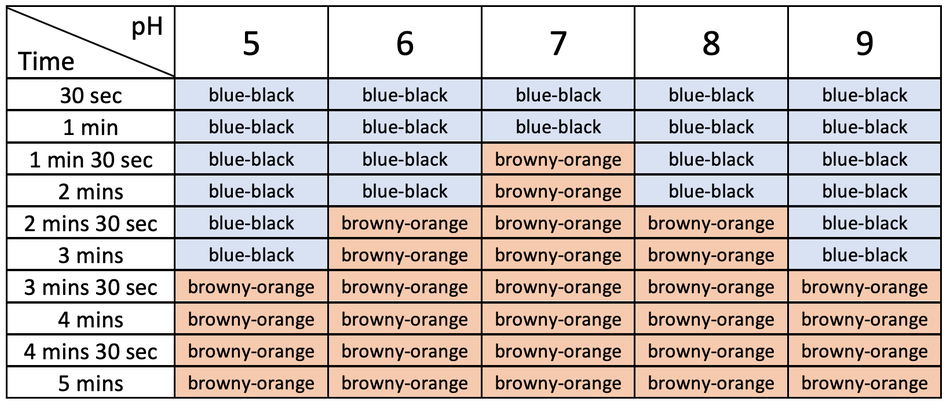
For each pH investigated, record the time taken for the disappearance of starch, ie when the iodine solution in the spotting tile remains orange-brown.
- Wear safety goggles.
- Amylase solution may cause allergic reactions.
- Iodine solution is irritant. Avoid contact with skin and eyes.
Example results
The time taken for the disappearance of starch is not the rate of reaction.
It will give us an indication of the rate, but it is the inverse close inverse The reciprocal. of the rate – the shorter the time taken, the greater the rate of the reaction.
We can calculate the rate of the reaction by dividing the number one by the time taken in seconds. The calculation is therefore \(\frac{1}{t}\).
For example, for the pH 5 investigation, the calculation would be:
1 ÷ 120 = 0.0083
So, from the results:
Plot a graph of rate of reaction against pH.
A similar experiment can be carried out to investigate the effect of temperature on amylase activity.
Set up a series of test tubes in the same way and maintain these at different temperatures using a water bath - either electrical or a heated beaker of water.
Depending on the chemical reaction under investigation, you might monitor the reaction in a different way. If investigating the effect of temperature on the breakdown of lipid close lipid Fat or oils, composed of fatty acids and glycerol. by lipase close lipase Enzyme that breaks down lipids (fats and oils). , for instance, you could monitor pH change – lipids are broken down into fatty acids close fatty acids Carboxylic acids with a long chain of carbon atoms. Fatty acids react with glycerol to produce lipids (fats and oils). and glycerol close glycerol Propane-1,2,3-triol. It reacts with fatty acids to form esters, found in nature as fats and oils. . As the reaction proceeded, the release of fatty acids would mean that the pH would decrease.
More guides on this topic
- Cell structures - OCR Gateway
- Respiration - OCR Gateway
- Photosynthesis - OCR Gateway
- Sample exam questions - cell level systems - OCR Gateway
Related links
- Biology: Exam-style questions
- Bitesize revision podcasts
- Personalise your Bitesize!
- Jobs that use Biology
- Save My Exams Subscription
- Tassomai Subscription
- Headsqueeze
- Revision Buddies Subscription

- GCSE Science
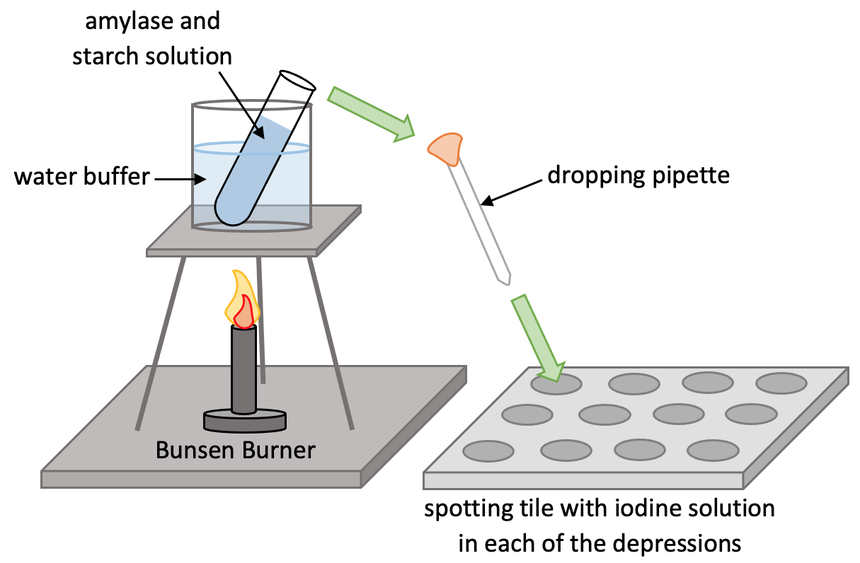
The next step is to place two boiling tubes in the beaker of water for around 5 minutes; one boiling tube will be filled with 5 cm 3 of amylase solution and the other will be filled with 5 cm 3 of starch suspension. The pH of these solutions will be 5 for the first experiment. These quantities will be the same throughout the whole experiment. We place the two separate test tubes in the beaker of water so that they both get up to 35°C.
- If the depressions in the spotting tile are brown-orange (the same colour as what iodine is), it tells us that no starch present, which means that the amylase enzyme has broken all of the starch down.
- If the depressions in the spotting tile are blue-black, it tells us that starch is still present, which means that the amylase enzyme has not broken all of the starch down yet.

IMAGES
COMMENTS
Pictured to the left is the Iodine and Benedict’s reagent which was used to perform the two tests. Picture 3. The picture to the left shows the results for the Iodine test. According to the results test tubes #2 and #4 are positive for the presence of starch while test tubes # 1,3, 5, and 6 are negative. Picture 4.
Repeat step 8 until the iodine no longer changes colour - meaning that there is no starch present, in other words the amylase has broken all starch down. Repeat steps 6-9 for each of the temperatures.
a Check the speed of the reaction with the suggested volumes of reactants to be used – 2 cm 3 of starch: 2 cm 3 of amylase: 1 cm 3 of buffer at pH 6. Ideally the reaction should take about 60 seconds at this pH: this is the usual optimum for amylase (see note 1). If the reaction is too fast, either reduce the enzyme volume or increase the ...
Jan 10, 2012 · iodine is added to a glucose solution, the only color seen is the red or yellow color of the iodine. Therefore, the faster the blue color of starch is lost, the faster the enzyme amylase is working. If the amylase is inactivated, it can no longer hydrolyze starch, so the blue color of the starch-iodine complex will persist.
e. Immediately at “time zero”, place 3 drops of amylase/starch solution in the first iodine-containing well. Record your observations. f. Repeat every 1 minute by placing 3 drops of starch/amylase mixture in a new iodine well and record your results. Continue until there is no color change (color stabilizes). 2. Test the effect of ...
In the reactions of amylase with starch, you will test for the disappearance of starch by reacting samples of the reaction mixtures with iodine. Initially, an unhydrolyzed starch solution reacts with iodine to give a blue-black color. The amylase catalyzes the hydrolysis of the -1,4 glycosidic linkages, forming maltose and dextrin.
ability of amylase to hydrolyze starch. We will detect the presence of starch in solution using iodine solution as an indicator. Iodine (I2) is a deep blue/black in the presence of starch. As starch is broken up to dextrins, the iodine turns to a brown/red color, followed by a pale brown/yellow when the enzyme has completed hydrolysis.
A control close control A part of the experiment in which all the ... for results with the enzyme to be valid close ... and test it with iodine solution; sample the starch-amylase mixture ...
The experiment uses iodine to test for the presence of starch. Iodine is a browny-orange colour. If starch is present, iodine will change from browny-orange to blue-black. If starch is not present, iodine will stay browny-orange. Before we start the experiment, we fill all of the depressions in the spotting tiles with iodine.
% starch solution into a test tube. 2. Measure 2 cm. 3. of 10 % amylase solution into a second test tube. 3. Place both tubes into a water bath set at 20 ºC for 3 minutes. 4. Place a drop of iodine in six wells of a spotting tile. 5. Remove both test tubes from the water bath. Pour the amylase into the starch/iodine solution and start the ...