An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

The Grandmother Effect: Implications for Studies on Aging and Cognition
James g herndon.
- Author information
- Article notes
- Copyright and License information
*James G. Herndon, PhD, Yerkes National Primate Research Center, Emory University, 954 Gatewood Rd. NE, Atlanta, GA 30329 (USA), Tel. +1 404 727 7752, Fax +1 404 727 3278, E-Mail [email protected]
Received 2009 Jan 22; Accepted 2009 Jun 10; Issue date 2010 Jan.
Women experience more years of vigorous life after ovulation has ceased than do females of other primate species. Is this an epiphenomenon of the greater life expectancy humans have enjoyed in the past century or so, or is long post-menopausal survival the result of an evolutionary selection process? Recent research implies the latter: Long post-menopausal survival came about through natural selection. One prominent line of thought explaining this selection process is the grandmother hypothesis .
To evaluate the implications of the hypothesis for non-human primate studies of aging and cognition.
The author presents a synopsis of the hypothesis, evaluates the uniqueness of the ‘grandmother effect’ to humans, and discusses its implications for non-human primate models of cognitive aging.
The hypothesis contends that, in past epochs, women who remained vigorous beyond their fertile years may have enhanced their reproductive success by providing care for their grandchildren. This care would have enabled their daughters to resume reproduction sooner, endowing them with greater lifetime fertility. Genes of grandmothers possessing such old-age vigor would be more likely to persist in subsequent generations. Is midlife menopause a uniquely human phenomenon, or does the chimpanzee, our closest primate relative, also display this trait? If so, we might expect a grandmother effect in this species as well. However, female chimpanzees continue to cycle until near the end of their maximum life span of about 60 years.
Long survival beyond fertility and a long life expectancy are distinctive human adaptations. The robustness of ancestral human grandmothers necessarily included resistance to cognitive decline through preservation of functions present in many primates but also development of processes of social cognition unique to our species. Cognitive traits such as language and social cognitive functions may function in our species in particular as mechanisms to compensate for age-related decline. This has significant implications for research in which non-human primates are considered as models of human cognitive aging; it also means that some processes can be studied only in humans.
Key Words: Grandmother hypothesis, Evolution, Aging and cognition, Non-human primates
Introduction
Women may enjoy many years of life after their childbearing careers have ended. One explanation as to how a long life span past fertility arose in our species is the grandmother hypothesis . This hypothesis holds that, in response to ecological changes at the Plio-Pleistocene boundary, there was reduced availability of food that human juveniles could handle themselves. In response to these factors the human female derived selective advantage for remaining robust as her fertility declined, enabling her to assist her daughters in provisioning their children [ 1 ]. The hypothesis proposes that life after menopause is not merely a result of mankind's success in postponing death through medical progress and changes in lifestyle, but rather that continued vigor after childbearing years is an evolved component of the human life cycle. This grandmother effect has several implications for aging. First, grandmothers’ prolonged vigor and ability to assist their offspring after they themselves are no longer capable of reproducing required concomitant evolution of resistance to age-related declines in vitality, including cognitive and social as well as physical robustness. Second, non-human species that do not display these characteristics present limitations as direct models of human aging processes.
In this essay, the author presents salient points of the argument that the long post-fertile life span of the human female is a derived trait that evolved along with mother-child food sharing and a long period of juvenile dependency [ 1 , 2 ]. Next, a controversy as to whether our closest living relative, the chimpanzee, experiences a period of extended post-menopausal infertility comparable to that of women is summarized. Finally, implications of the grandmother effect for research on age-related changes in cognition are discussed.
The Grandmother Hypothesis
A key question in theories of aging is why the life histories of animal species vary in terms of the relative amount of time spent during development and reproduction, and how these patterns may relate to aging. One important factor appears to be environmental risk of mortality. Charnov [ 2 ] argues that environments with low mortality risk provide more time to invest in growth and development as well as longer life. A related view is that of the ‘disposable soma’, which holds that there is a tradeoff between investment in the soma and the investment in reproduction, based upon risks in the environment [ 3 ]. In this context, long-lived primate species are examples of a relatively greater investment in soma.
These ideas help explain why primates are long-lived, but why does Homo sapiens have such a long life span, even among primates? One view as to how selection pressure could change life history was provided by the ‘stopping early’ or ‘mother’ hypothesis, proposed by Williams [ 4 ]. He argued that menopause was a benefit to a woman because a ‘… termination of increasingly hazardous pregnancies would enable her to devote her whole remaining energy to the care of her living children, and would remove childbirth mortality as a cause for failure to raise these children’.
In contrast to this stopping early hypothesis, the grandmother hypothesis considers ovarian aging and menopause to be a conserved human trait, rather than a derived one, present before the branching off of the human lineage. It considers the derived trait to be slower aging in other physiological systems, resulting in a longer life span. A number of reviews of this hypothesis and its implications have appeared; it is briefly outlined here as it was put forth by Hawkes [ 5 , 6 , 7 ], one of its principal architects.
The grandmother hypothesis posits that ancestral females benefitted by retaining their strength as their fertility declined, enabling them to assist their daughters in caring for their children. The benefit of her longer survival was an increase in her daughters’ fertility. Hawkes [ 5 ] argues that this change came about because ecological shifts forced dependence on foods that weanlings could not handle effectively. This presented an opportunity for an older female whose own fertility was ending to increase her own fitness by provisioning her grandchildren. According to this model, grandmothering explains increased adult survival and a longer life span. This, in turn, favors a longer period of prepubertal development [ 8 ].
A wide range of evidence establishes the plausibility of this concept. One line of support stems from observations of both modern-day traditional societies and historical accounts of pre-industrial Western societies. Among the Hadza, for example, one dietary staple are deep-growing roots that children have difficulty harvesting. Post-menopausal women, however, are able to dig them up, thus contributing to improved nutrition of their grandchildren [ 9 ]. Hawkes [ 5 ] points out that harsher, drier environmental conditions that appeared in the Plio-Pleistocene transition may have thus provided a ‘novel fitness opportunity’ for older women. She posits that these older females may have exploited this opportunity by helping to feed their just-weaned grandchildren, thus allowing their daughters to reproduce sooner without compromising the welfare of those weanlings [ 9 ]. The food-sharing that is the basis of the grandmother hypothesis may have involved other foods besides tubers that were too difficult for a child to handle, such as small game, shellfish, nuts, or seeds, as long as these were available in sufficient quantity in a particular ecological setting [ 1 ]. In addition to Hawkes’ observations on the Hadza, several other studies describe societies in which work by grandmothers contributes to the material support of their daughters’ weanlings in both modern [ 10 ] and historical populations [ 11 , 12 ], as reviewed in Sear and Mace [ 13 ].
Does grandmothering indeed increase ultimate fertility? This question was addressed by Lahdenperä et al. [ 14 ], who analyzed archival data on longevity and reproduction of pre-industrial populations of about 500 Finnish and 2,300 Canadian women. Their primary finding in both of these groups was that the longer women lived after their childbearing years (i.e., after 50 years), the greater was the number of their grandchildren (produced by both sons and daughters). The Finnish dataset showed, in addition, that those young women living near to their mother began reproducing earlier and had greater lifetime reproductive success than did the daughters of women who were deceased or resided in a different village. Thus, at least in these two societies, the longevity of grandmothers is associated with increased number of descendants, suggesting that post-menopausal longevity indeed confers a selection advantage.
Do Women Have Uniquely Long Lives after Childbearing Has Ceased?
If this trait indeed evolved in the rising hominins, then Homo sapiens should be the only living species to display it. In a recent review of the literature on menopause in non-human primates, Walker and Herndon [ 15 ] observed that age-dependent cessation of ovulation occurs in all primate species studied. Rhesus monkeys, for example, reach menopause around 25–30 years of age, which is well beyond their median survival of about 16 years and near the maximum life span of about 35 years [ 16 ]. Thus, it is not menopause, but a lengthy post-menopausal life span that is lacking in monkeys. But if one accepts the scenario of working grandmothers improving their daughters’ fertility, the question remains whether menopause evolved as a way of stopping reproduction, or whether longevity past menopause was the adaptation. In this regard, the chimpanzee is of particular interest as the species biologically most closely related to humans. In captivity, the median life span of this species is about 26 years and the maximum about 60 years 1 [ 18 ]. Thus, the question is whether the chimpanzee's life history follows a ‘non-human primate’ pattern, with fertility declining in synchrony with somatic aging, or whether it exhibits a ‘humanized’ pattern, with menopause and reproductive cessation earlier in life so that a long post-reproductive period ensues.
In the case of the chimpanzee, it has been difficult to distinguish between these two schedules because of the scarcity of lifelong observations of their reproductive status. But the data available show that they continue to ovulate until near death [ 19 , 20 , 21 ], suggesting that chimpanzees, like other simians, follow the non-human pattern. However, a later report concluded that menopause occurs near mid-life, as it does in women. This later study on 14 chimpanzees found that FSH rose above what the authors designated as a critical level between 36 and 40 years of age [ 22 ] and concluded that menopause in chimpanzees occurred between 35 and 40 years of age. Although this study did follow individuals longitudinally, the authors were unable to observe actual menstrual cycles, relying instead upon twice-yearly samples of gonadotrophic hormones. Menstrual bleeding was not regularly observed in the chimpanzee facility.
Since menstrual bleeding has been observed regularly in many chimpanzees at the colony of the Yerkes National Primate Research Center for several decades, we examined the records for evidence of cyclic menstrual bleeding to directly track cycles and to determine when menopause occurs. Data were available for 664 chimpanzee-years of observation on 89 chimpanzees, from the ages of 6 to 59 years. Twenty of the chimpanzees were observed into their 40th year of life (an age rarely achieved in chimpanzees in the wild or even in captivity [ 18 , 23 ]), and all of these showed cycles demarcated by menstrual bleeding after the age of 39 years. Three of the chimpanzees were observed into their 50s; all 3 showed cycles of menstrual bleeding past that age. We observed apparent menopause (defined as cessation of cycles for a period of at least 12 months without any underlying deterioration in overall health [ 24 ]) in only 1 chimpanzee. This individual ceased to display menstrual bleeding at 53 years of age. She stopped monthly cycles of genital swelling (which is dependent upon cycles of ovarian hormones [ 25 , 26 , 27 , 28 , 29 , 30 ]) at 57 years of age and lived in relatively good health until her death at 59 years [ 31 ].
While our data on chimpanzees clearly demonstrate that menstrual cycles can continue well past the age of 40 years and even into the 50s, one might still question whether the observed cycles are reproductively viable. However, a study of six groups of chimpanzees living in the wild indicated that live births occur at maternal ages as late as 40 or 50 years [ 23 ]. Late-life deliveries also can occur in captivity, as in a recent case report of a chimpanzee documented to be 49 years of age who gave birth to a healthy infant in a zoo in Switzerland [ 32 ].
Given these data and our finding that menstrual cycles can persist into the 50s, it seems that the chimpanzee does not possess lengthy, human-like post-menopausal survival. It therefore appears that a long and vigorous life, extending many years past the end of fertility, is a distinctive human trait.
It may be tempting to think that this human trait is merely an artifact of the lengthening of median human life expectancy in the last centuries. Extensive data, however, indicate that menopause and a long post-cycling life span have not emerged recently. Vaupel et al. [ 33 ], for example, noted that in a Swedish farming population, the death rate for women below the age of 70 years remained at a low and nearly constant level of about 0.05 throughout the period from 1875 through 1950. A high probability of adult survival past menopause has also been described in other historical populations as well as in human foraging societies [as reviewed in [ 5 ]]. There are ancient references to menopause as well [see [ 34 ]]. This echoes the commonplace observation that, throughout history, at least some women have lived to an advanced old age, well beyond the termination of fertility.
Is Old-Age Cognition Different in Humans?
It appears, therefore, that the long human life span is an evolutionary adaptation of our species. This adaptation must have involved maintenance of physical robustness of aged individuals, as in the case of the tuber-digging Hadza grandmothers [ 9 ]. The brain also must have evolved improved resistance to decline. Finch and Sapolsky [ 35 ] propose that this may have included metabolic changes that protect against Alzheimer disease-like pathology. Their proposal also implies that the human line developed general resistance against failing cognitive abilities. In the absence of enhanced cognitive resilience, extended life span may have been impossible, as failing brain function would likely cause increased mortality from environmental hazards, whereas preserved memory and decision-making ability would clearly be instrumental to survival. Indeed, Allen et al. [ 36 ] have suggested that increased brain size and greater cognitive capacity supported the development of increased life span in humans. They relate this brain-driven increase to the phenomenon of ‘cognitive reserve’, which compensates for brain lesions and pathology through excess capacity [ 37 ]. While Allen et al. [ 36 ] suggest a causal link between increased cognitive ability and long life span, an alternative view is that larger brains evolved because of the increased demands of an intercorrelated group of traits that included later age at maturity, longer life span, and large and complex social groups. The importance of social complexity for brain size is emphasized by Dunbar [ 38 ] in his ‘social brain’ hypothesis.
If the grandmother hypothesis indeed explains our long life span, then human late-life robustness – and decline – must be considered distinctively human. This implies that many aspects of cognitive decline can be studied only in humans, not in an animal model. Behaviors involving language, such as word generation or verbal memory, are obvious examples. In addition, other characteristic human abilities and behaviors may have been elaborated through grandmothering. For example, Carstensen and Lockenhoff [ 39 ] argue that other behaviors of grandmothers (and grandfathers) besides assistance with provisioning of weaned but dependent children may also have led to longer survival. They think that ‘older kin may have contributed to the survival of younger individuals by providing instrumental support, knowledge, social expertise, and conflict resolution’ and that the genes of older individuals making these investments in younger individuals are more likely to survive in subsequent generations. Some of these behaviors and abilities, they propose, improve, rather than decline, with age.
The uniqueness of some aspects of human aging does not lessen the importance of studying aging (and cognitive decline) in non-human primates; in fact, it emphasizes the importance of identifying and understanding the physical and behavioral traits that make us unique. Many studies of cognitive aging, including our own, have focused on non-human primates. Most of this work has been conducted on the rhesus monkey, rather than the chimpanzee. This species, it has been argued, shares many features of cognitive function with humans. In fact, humans and rhesus monkeys both display age-related decline in many of the same cognitive tasks. Both species are characterized by great variability in the display of cognitive loss, with some individuals displaying only modest impairment, even at advanced ages [ 40 , 41 ]. To the extent that the rhesus monkey is an apt model of human aging, it also can provide a window upon neural mechanisms of the aging process, since the anatomy and physiology of this species can be studied more readily than in humans.
In this context, the chimpanzee represents an appropriate contrast with both the human and the widely studied rhesus monkey. With an average adult brain weight of about 385 g [ 18 ], the chimpanzee has about four times as much brain mass as the rhesus monkey (at 90 g [ 42 ]) and one-fourth the mass of the human (at about 1,300 g [ 43 ]); hence, the chimpanzee occupies a unique position in the animal kingdom with respect to humans and other non-human primates. The phylogenetic importance of the comparison with humans is underscored by the fact that the chimpanzee's brain, body size, and maturational pattern are similar to those of australopithocenes, the genus ancestral to Homo [ 5 ].
The comparison of these three species can offer information of practical benefit. Although we know that the rhesus monkey exhibits many of the same cognitive deficits that befall humans, we know little about cognitive decline in the chimpanzee. Does the fourfold advantage in brain mass over the monkey provide the chimpanzee with particular resistance to cognitive decline? There have been very few studies on the cognitive capacity of aged chimpanzees, but the available data suggest that the chimpanzee may actually be resistant to decline in one classic test of age-related decline in frontal lobe function: the delayed-recognition task . In this task, a treat or (for humans) another object is hidden in one of two locations while the subject looks on. Later, after a variable delay, the two hiding places are presented again. Normally, aged human and monkey subjects have difficulty remembering the location of the reward, and this difficulty increases with increased delay. Further, the effect of increasing delay has a disproportionate impact on aged persons and monkeys [ 44 ]. In the only study using this paradigm in 19 chimpanzees (aged 7–41 years), however, there was an advantage for the younger subjects only at the very shortest delay. As delays increased to a point where older monkeys began to be impaired, the older chimpanzees actually performed as well as the young [ 45 ]. While this early study was well carried out, it awaits replication to determine if chimpanzees are uniquely resistant to this age-related change. Indeed, the finding that large brains are linearly correlated with longer life spans in primates [ 46 ] does suggest this possibility. But why do humans show an age-related deficit on this task? This question can only be answered by studies comparing chimpanzees and humans.
Figure 1 brings into sharp focus the contrast in life history among females of the three primate species discussed here. Rhesus monkeys live shorter lives than chimpanzees or women, with menopause occurring only in those few monkeys whose life spans approach the species’ maximum. (Note that the median life expectancy in rhesus is only about 16 years.) Women and chimpanzees experience similar rates of ovarian aging [ 47 ] so that both species stop ovulating at about 50 years. However, most chimpanzees die before menopause, while most women survive well beyond. Physical strength, cognitive resilience, and social adaptability all play an important role in this peculiarly human phenotype. This human uniqueness increases the urgency of studying aging in other primates, an urgency heightened by the declining numbers and endangered status of chimpanzees and other apes. One reason for this is that the decline seen in other species may be accelerated at a rate proportionate to their relatively ‘faster’ life histories. Likewise, unique aspects of human brain morphology at molecular, cellular, and anatomical levels may provide clues to nervous system adaptations that have conferred a uniquely long life span upon humans. These same insights may help explain our susceptibility to such uniquely human scourges as Alzheimer, Huntington, and Parkinson diseases.
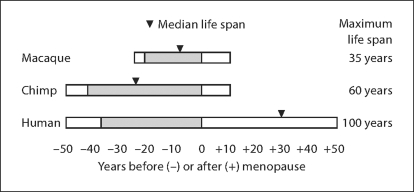
Schematic diagram depicting approximate ages at puberty, menopause, and maximum life span of female macaques (Macaca spp.) , chimpanzees (Pan troglodytes) , and humans (Homo sapiens) . Bars are aligned to the age at menopause; shading indicates the fertile time span between onset of puberty and menopause. Approximate ages used are based upon references [ 15 , 16 , 18 , 48 , 49 ] : puberty: 3.5, 8, 12.5 years; median life span: 16, 26, 80 years; menopause: 25, 50, 50 years; life span: 35, 60, 100 years. The age of 100 years was arbitrarily chosen for the human life span because survival to this age is very rare; only about 2 years of life expectancy remain in 20th century European and North American cohorts [ 50 ].
Acknowledgements
The author thanks Johannes Tigges, Agnès Lacreuse, Margarete Tigges, Kathleen Herndon, Lara Kristin Lentini, Lary Walker, Margaret Walker, Todd Preuss, James Rilling, Doris Jane Langford, Kristen Hawkes, and two anonymous reviewers for comments on the manuscript. Supported by National Institutes of Health grants P51RR000165 and P01AG026423.
The popular press has presented claims that Cheeta, the chimpanzee in the Tarzan movies, has reached the age of 76 years. However, a recent report by a journalist who was preparing a biography of this supposedly superannuated chimpanzee found that Cheeta was actually around 45 years of age in 2008, and the anecdotes supporting his advanced age were apocryphal [ 17 ].
- 1. O'Connell J, Hawkes K, Blurton Jones NG. Grandmothering and the evolution of Homo erectus. J Hum Evol. 1999;36:461–485. doi: 10.1006/jhev.1998.0285. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Charnov EL. Life History Invariants: Some Explorations of Symmetry in Evolutionary Ecology. Oxford: Oxford University Press; 1993. [ Google Scholar ]
- 3. Kirkwood TBL, Rose MR. Evolution of senescence: late survival sacrificed for reproduction. Philos Trans Biol Sci. 1991;332:15–24. doi: 10.1098/rstb.1991.0028. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [ Google Scholar ]
- 5. Hawkes K. Grandmothers and the evolution of human longevity. Am J Hum Biol. 2003;15:380–400. doi: 10.1002/ajhb.10156. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Hawkes K. Life history and human evolution: a chronicle of ideas and findings. In: Hawkes K, Paine RR, editors. The Evolution of Human Life History. Santa Fe: School of American Research Press; 2006. pp. 45–94. [ Google Scholar ]
- 7. Hawkes K. Slow life histories and human evolution. In: Hawkes K, Paine RR, editors. The Evolution of Human Life History. Santa Fe: School of American Research Press; 2006. pp. 95–126. [ Google Scholar ]
- 8. Hawkes K, O'Connell JF, Blurton Jones NG. Human life histories: primate tradeoffs, grandmothering socioecology, and the fossil record. In: Kappeler P, Pereira M, editors. Primate Life Histories and Socioecology. Chicago: University of Chicago Press; 2003. pp. 204–227. [ Google Scholar ]
- 9. Hawkes K, O'Connell JF, Blurton Jones NG. Hadza women's time allocation, offspring provisioning, and the evolution of long postmenopausal life spans. Curr Anthropol. 1997;38:551–577. [ Google Scholar ]
- 10. Sear R, Mace R, McGregor IA. Maternal grandmothers improve nutritional status and survival of children in rural Gambia. Proc Biol Sci. 2000;267:1641–1647. doi: 10.1098/rspb.2000.1190. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Jamison CS, Cornell LL, Jamison PL, Nakazato H. Are all grandmothers equal? A review and a preliminary test of the ‘grandmother hypothesis’ In Tokugawa, Japan. Am J Phys Anthropol. 2002;119:67–76. doi: 10.1002/ajpa.10070. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Voland E, Beise J. Opposite effects of maternal and paternal grandmothers on infant survival in historical Krummhörn. Behav Ecol Sociobiol. 2002;52:435–443. [ Google Scholar ]
- 13. Sear R, Mace R. Who keeps children alive? A review of the effects of kin on child survival. Evol Hum Behav. 2008;29:1–18. [ Google Scholar ]
- 14. Lahdenperä M, Lummaa V, Helle S, Tremblay M, Russell AF. Fitness benefits of prolonged post-reproductive lifespan in women. Nature. 2004;428:178–181. doi: 10.1038/nature02367. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Walker ML, Herndon JG. Menopause in nonhuman primates? Biol Reprod. 2008;79:398–406. doi: 10.1095/biolreprod.108.068536. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Tigges J, Gordon TP, McClure HM, Hall EC, Peters A. Survival rate and life span of rhesus monkeys at the Yerkes Regional Primate Research Center. Am J Primatol. 1988;15:263–273. doi: 10.1002/ajp.1350150308. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Rosen RD: Lie of the Jungle: The Truth about Cheeta the Chimpanzee. Washington Post, Washington, DC, December 14, 2008.
- 18. Herndon JG, Tigges J, Anderson DC, Klumpp SA, McClure HM. Brain weight throughout the life span of the chimpanzee. J Comp Neurol. 1999;409:567–572. [ PubMed ] [ Google Scholar ]
- 19. Bellino FL, Wise PM. Nonhuman primate models of menopause workshop. Biol Reprod. 2003;68:10–18. doi: 10.1095/biolreprod.102.005215. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Graham CE. Reproductive function in aged female chimpanzees. Am J Phys Anthropol. 1979;50:291–300. doi: 10.1002/ajpa.1330500302. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Gould KG, Flint M, Graham CE. Chimpanzee reproductive senescence: a possible model for the evolution of menopause. Maturitas. 1981;3:157–166. doi: 10.1016/0378-5122(81)90007-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Videan EN, Fritz J, Heward CB, Murphy J. The effects of aging on hormone and reproductive cycles in female chimpanzees (Pan troglodytes) Comp Med. 2006;56:291–299. [ PubMed ] [ Google Scholar ]
- 23. Emery Thompson M, Jones JH, Pusey AE, Brewer-Marsden S, Goodall J, Marsden D, Matsuzawa T, Nishida T, Reynolds V, Sugiyama Y, Wrangham RW. Aging and fertility patterns in wild chimpanzees provide insights into the evolution of menopause. Curr Biol. 2007;17:2150–2156. doi: 10.1016/j.cub.2007.11.033. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Burger HG. The endocrinology of the menopause. J Steroid Biochem Mol Biol. 1999;69:31–35. doi: 10.1016/s0960-0760(98)00145-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Deschner T, Heistermann M, Hodges K, Boesch C. Female sexual swelling size, timing of ovulation, and male behavior in wild West African chimpanzees. Horm Behav. 2004;46:204–215. doi: 10.1016/j.yhbeh.2004.03.013. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Graham CE, Collins DC, Robinson H, Preedy JR. Urinary levels of estrogens and pregnanediol and plasma levels of progesterone during the menstrual cycle of the chimpanzee; relationship to the sexual swelling. Endocrinology. 1972;91:13–24. doi: 10.1210/endo-91-1-13. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Dahl J, Nadler RD, Collins DC. Monitoring the ovarian cycles of Pan troglodytes and P. paniscus: a comparative approach. Am J Primatol. 1991;24:195–209. doi: 10.1002/ajp.1350240306. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Nadler RD, Graham CE, Gosselin R, Collins DC. Serum levels of gonadotropins and gonadal steroids, including testosterone, during the menstrual cycle of the chimpanzee (Pan troglodytes) Am J Primatol. 1985;9:273–284. doi: 10.1002/ajp.1350090404. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. McArthur JW, Beitens IZ, Gorman A, Collins DC, Preedy JRK, Graham CE. The interrelationships between sex skin swelling and the urinary excretion of LH, estrone, and pregnanediol by the cycling female chimpanzee. Am J Primatol. 1981;1:265–270. doi: 10.1002/ajp.1350010303. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Emery Thompson M. Reproductive endocrinology of wild female chimpanzees (Pan troglodytes schweinfurthii): methodological considerations and the role of hormones in sex and conception. Am J Primatol. 2005;67:137–158. doi: 10.1002/ajp.20174. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Lacreuse A, Chennareddi L, Gould KG, Hawkes K, Wijayawardana SR, Chen J, Easley KA, Herndon JG. Menstrual cycles continue into advanced old age in the common chimpanzee (Pan troglodytes) Biol Reprod. 2008;79:407–412. doi: 10.1095/biolreprod.108.068494. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Puschmann W, Federer E. Ein neuer Fertilitätsnachweis bei einer hoch betagten Schimpansin und Anmerkungen zum Höchstalter von Pan troglodytes. Zool Gart. 2008;77:182–185. [ Google Scholar ]
- 33. Vaupel JW, Carey JR, Christensen K, Johnson TE, Yashin AI, Holm NV, Iachine IA, Kannisto V, Khazaeli AA, Liedo P, Longo VD, Zeng Y, Manton KG, Curtsinger JW. Biodemographic trajectories of longevity. Science. 1998;280:855–860. doi: 10.1126/science.280.5365.855. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Peccei JS. Menopause: adaptation or epiphenomenon? Evol Anthrop. 2001;10:43–57. [ Google Scholar ]
- 35. Finch CE, Sapolsky RM. The evolution of Alzheimer disease, the reproductive schedule, and apoeE isoforms. Neurobiol Aging. 1999;20:407–428. doi: 10.1016/s0197-4580(99)00053-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Allen JS, Bruss J, Damasio H. The aging brain: the cognitive reserve hypothesis and hominid evolution. Am J Hum Biol. 2005;17:673–689. doi: 10.1002/ajhb.20439. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [ PubMed ] [ Google Scholar ]
- 38. Dunbar R. The social brain hypothesis. Evol Anthrop. 1998;6:179–190. [ Google Scholar ]
- 39. Carstensen LL, Lockenhoff CE. Aging, emotion, and evolution: The bigger picture. Ann NY Acad Sci. 2003;1000:152–179. doi: 10.1196/annals.1280.008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Herndon JG, Moss MB, Rosene DL, Killiany RJ. Patterns of cognitive decline in aged rhesus monkeys. Behav Brain Res. 1997;87:25–34. doi: 10.1016/s0166-4328(96)02256-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Moss MB, Moore TL, Schettler SP, Killiany RJ, Rosene DL. Successful versus unsuccessful aging in the rhesus monkey. In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. Boca Raton: CRC Press; 2007. pp. 21–38. [ PubMed ] [ Google Scholar ]
- 42. Herndon JG, Tigges J, Klumpp SA, Anderson DC. Brain weight does not decrease with age in adult rhesus monkeys. Neurobiol Aging. 1998;19:267–272. doi: 10.1016/s0197-4580(98)00054-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Hartmann P, Ramseier A, Gudat F, Mihatsch MJ, Polasek W. Das Normgewicht des Gehirns beim Erwachsenen in Abhängigkeit von Alter, Geschlecht, Körpergrösse und Gewicht. Pathologe. 1994;15:165–170. doi: 10.1007/s002920050040. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Rapp PR, Amaral DG. Evidence for task-dependent memory dysfunction in the aged monkey. J Neurosci. 1989;9:3568–3576. doi: 10.1523/JNEUROSCI.09-10-03568.1989. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Riopelle AJ, Rogers CM. Age changes in chimpanzees. In: Schrier AM, Stolnitz F, editors. Behavior of Nonhuman Primates: Modern Research Trends. New York: Academic Press; 1965. pp. 449–462. [ Google Scholar ]
- 46. Judge DS, Carey JR. Postreproductive life predicted by primate patterns. J Gerontol A Biol Sci Med Sci. 2000;55:B201–B209. doi: 10.1093/gerona/55.4.b201. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Jones KP, Walker LC, Anderson D, Lacreuse A, Robson SL, Hawkes K. Depletion of ovarian follicles with age in chimpanzees: similarities to humans. Biol Reprod. 2007;77:247–251. doi: 10.1095/biolreprod.106.059634. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Hawkes K, Smith K, Robson S. Mortality and fertility rates in humans and chimpanzees: how within-species variation complicates cross-species comparisons. Am J Hum Biol. 2009;21:578–586. doi: 10.1002/ajhb.20890. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 49. Oeppen J, Vaupel JW. Demography. Broken limits to life expectancy. Science. 2002;296:1029–1031. doi: 10.1126/science.1069675. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Database HM: University of California, Berkeley (USA), and Max Planck Institute for Demographic Research (Germany). Available at www.mortality.org or www.humanmortality.de (data downloaded on January 5, 2009).
- View on publisher site
- PDF (129.7 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- News & Views
- Published: 11 March 2004
Human longevity
The grandmother effect
- Kristen Hawkes 1
Nature volume 428 , pages 128–129 ( 2004 ) Cite this article
25k Accesses
148 Citations
491 Altmetric
Metrics details
Why do women live long past the age of child-bearing? Contrary to common wisdom, this phenomenon is not new, and is not due to support for the elderly. Rather, grannies have a lot to offer their grandchildren.
This is a preview of subscription content, access via your institution
Relevant articles
Open Access articles citing this article.
Adaptation across the Lifespan: Towards a Processual Evolutionary Explanation of Human Development
- Werner Greve
Integrative Psychological and Behavioral Science Open Access 25 April 2023
Grandparental Support and Maternal Postpartum Mental Health
- Madelon M.E. Riem
- , Marian J. Bakermans-Kranenburg
- … Marinus H. van IJzendoorn
Human Nature Open Access 08 February 2023
Turnover in close friendships
- Chandreyee Roy
- , Kunal Bhattacharya
- … Kimmo Kaski
Scientific Reports Open Access 30 June 2022
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

POPPERFOTO.COM
Lahdenperä, M., Lummaa, V., Helle, S., Tremblay, M. & Russell, A. F. Nature 428 , 178–181 (2004).
Article ADS Google Scholar
Oeppen, J. & Vaupel, J. W. Science 296 , 1029–1031 (2002).
Article CAS Google Scholar
Nishida, T. et al. Am. J. Primatol. 59 , 99–121 (2003).
Article Google Scholar
Hill, K. et al. J. Hum. Evol. 39 , 1–14 (2001).
Google Scholar
Kaplan, H. K. Pop. Dev. Rev. 20 , 753–791 (1994).
Harvey, P. H., Read, A. F. & Promislow, D. E. L. Oxford Surv. Evol. Biol. 6 , 13–31 (1989).
Ricklefs, R. E. Am. Nat. 152 , 24–44 (1998).
Charnov, E. L. Life History Invariants: Some Explorations of Symmetry in Evolutionary Ecology (Oxford Univ. Press, 1993).
Alvarez, H. P. Am. J. Phys. Anthropol. 133 , 435–450 (2000).
Goodall, J. The Chimpanzees of Gombe (Harvard Univ. Press, Cambridge, MA, 1986).
O'Connell, J. F., Hawkes, K. & Blurton Jones, N. G., J. Hum. Evol. 36 , 461–485 (1999).
Lee, R. D. Proc. Natl Acad. Sci. USA 100 , 9637–9642 (2003).
Article ADS CAS Google Scholar
Hawkes, K. Am. J. Hum. Biol. 15 , 380–400 (2003).
Hawkes, K., O'Connell, J. F. & Blurton Jones, N. G. in Primate Life Histories and Socioecology (eds Kappeler, P. & Pereira, M.) 204–227 (Univ. Chicago Press, 2003).
Doblhammer, G. & Oeppen, J. Proc. R. Soc. Lond. B 270 , 1541–1547 (2003).
Ricklefs, R. E. & Wikelski, M. Trends Ecol. Evol. 17 , 462–468 (2002).
Coale, A. J. & Demeny, P. Regional Model Life Tables and Stable Populations 2nd edn (Princeton Univ. Press, 1983).
Download references
Author information
Authors and affiliations.
Department of Anthropology, University of Utah, 270 S. 1400 E. Stewart 102, Salt Lake City, 84102, Utah, USA
Kristen Hawkes
You can also search for this author in PubMed Google Scholar
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Hawkes, K. The grandmother effect. Nature 428 , 128–129 (2004). https://doi.org/10.1038/428128a
Download citation
Issue Date : 11 March 2004
DOI : https://doi.org/10.1038/428128a
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Unraveling female reproductive senescence to enhance healthy longevity.
- Daniel Boon Loong Teh
- Zhongwei Huang
Cell Research (2023)
Integrative Psychological and Behavioral Science (2023)
- Marian J. Bakermans-Kranenburg
- Marinus H. van IJzendoorn
Human Nature (2023)
- Kunal Bhattacharya
- Kimmo Kaski
Scientific Reports (2022)

Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
Increased longevity evolves from grandmothering
Peter s kim, james e coxworth, kristen hawkes.
- Author information
- Article notes
- Copyright and License information
Author for correspondence ( [email protected] ).
Received 2012 Jul 27; Accepted 2012 Oct 2; Issue date 2012 Dec 22.
Postmenopausal longevity may have evolved in our lineage when ancestral grandmothers subsidized their daughters' fertility by provisioning grandchildren, but the verbal hypothesis has lacked mathematical support until now. Here, we present a formal simulation in which life spans similar to those of modern chimpanzees lengthen into the modern human range as a consequence of grandmother effects. Greater longevity raises the chance of living through the fertile years but is opposed by costs that differ for the sexes. Our grandmother assumptions are restrictive. Only females who are no longer fertile themselves are eligible, and female fertility extends to age 45 years. Initially, there are very few eligible grandmothers and effects are small. Grandmothers can support only one dependent at a time and do not care selectively for their daughters' offspring. They must take the oldest juveniles still relying on mothers; and infants under the age of 2 years are never eligible for subsidy. Our model includes no assumptions about brains, learning or pair bonds. Grandmother effects alone are sufficient to propel the doubling of life spans in less than sixty thousand years.
Keywords: human evolution, life history, sexual conflict
1. Introduction
Female fertility ends at similar ages in humans and the other great apes; all can have latest deliveries into the forties but not beyond [ 1 ]. But other ape females become frail in their thirties [ 2 ] and usually die during the cycling years. This is not true of humans. Even among hunter–gatherers, women past the childbearing years make up substantial fractions of human populations [ 3 – 6 ]. These comparisons suggest that the ancestral age when fertility ends has persisted among all great apes, while greater longevity evolved in our lineage. As W. D. Hamilton noted, the mismatch between human longevity and female fertility ‘inevitably suggests the special value of the old woman as mother or grandmother during a long ancestral period’ [ 7 , p. 37]. Subsequent evidence from hunter–gatherers pointed to the special value of grandmothers supplying foods that just weaned juveniles cannot acquire effectively for themselves [ 8 ]. This economic productivity of older women prompted the Grandmother Hypothesis.
A verbal scenario begins with changing ecology. Increasingly arid and seasonal PlioPleistocene African savannahs constricted the distribution of foods ancestral juveniles could handle. This left ancestral mothers two choices: follow retreating foods and maintain diets their weanlings could manage or subsidize their offspring to older ages. Longer dependence would delay mothers' next birth, but also present a novel fitness opportunity to older females whose own fertility was declining. Elders could compensate for increased juvenile dependence by helping their grandchildren, allowing their daughters to have another baby sooner without risking the survival of previous offspring. Vigorous grandmothers could help more and leave more descendants. Consequently, longevity would have increased, expanding the fraction of female years lived past the fertile ages [ 9 – 11 ]. Or would it?
Researchers have looked for, and usually found, evidence of grandmother effects in contemporary and historical human populations (e.g. [ 12 – 14 ] but see [ 15 ]). But contributions by elders to the welfare of their younger kin might be consequences of postmenopausal life spans that evolved for other reasons. The question remains whether grandmother effects could transform a great ape-like life history in which adult females usually die during the cycling years into a human life history in which they usually do not.
Approaches to the mismatch between the end of female fertility and survival in humans often pose the question as the puzzle of menopause not increased longevity. This follows Williams' [ 16 ] influential formulation that assumed menopause to be unique to humans. This assumption is now known to be false [ 17 , 18 ]. Williams' focus on when to stop, generally taking observed rates of human ageing and population age structure as givens, has stimulated several treatments of the evolution of menopause, formal and otherwise (e.g. [ 4 , 19 – 21 ]).
Only recently has the evolution of postmenopausal longevity begun to receive formal attention. Lee [ 22 ] considered the effects of intergenerational transfers on selection against senescence. In contrast to Lee, we begin with an ancestral ape-like condition and include two sexes. Kachel et al. [ 23 ] used the Grandmother Hypothesis as their guide to construct an agent-based model of helpful grandmother effects on the evolution of lifespans. Our subsequent analysis of their model [ 24 , 25 ] set the foundation for the model we report here. In contrast to Kachel and others, we begin with a model population that is at an ape-like equilibrium for longevity without grandmothering, and ask whether weak grandmother effects could propel the evolution of increased longevity.
2. Model and results
We simulated the model in box 1 , with parameters in table 1 , using M atlab R (2011 b ). A key model parameter is L , the expected adult life span. Because we use the simplifying assumption that mortality is constant (see the electronic supplementary material for further discussion of the consequences of this assumption), L is the inverse of the annual mortality rate and does not change with age . Guided by demographic data from other great apes (see the electronic supplementary material), we specified expected adult life spans ranging from 16 to 27 years to represent the ancestral condition. Using findings from three well-known hunter–gatherer groups (Dobe !Kung [ 3 ], forest-dwelling Ache [ 4 ], Hadza [ 5 ]), we set an expected adult life span of 43 years as our human target. For starting populations, we assumed that the system begins with 1000 individuals, half male and half female, all of whom have the same expected adult life span, L . For convenience, we specified that the ages of individuals are distributed uniformly from τ 1 ( L ) to τ 3 . From this distribution, the population converges to a steady-state age distribution within several generations.
Box 1. Mathematical model.
Here, we describe a probabilistic agent-based model, which we will then convert to a deterministic difference equations model (see the electronic supplementary material). The agent-based model has the following features.
Mortality . For simplicity, we assume mortality rates are constant. Each individual has a lifetime mortality rate 1/ L , where L is the individual's expected life span from any age, including the beginning of adulthood. We refer to L as the expected adult life span because this is our main interest (see the electronic supplementary material for discussion of the constant mortality assumption). In addition, the population is subject to an extrinsic, population-dependent death rate that affects everyone equally. Calculation of the population-dependent death rate is explained in the Agent-based model algorithm described in the electronic supplementary material.
Longevity trade-offs . Greater expected adult life span ( L ) always increases the chance of living through the fertile ages, but as is typical of mammals [ 26 ], females with greater longevity have offspring that take longer to reach independence. We assume age at independence to be L /6. Males with greater expected adult life spans are less successful at competing for paternities following Williams' [ 16 ] deduction that selection for reduced senescence should decrease youthful vigour. Our male fertility–longevity trade-off assigns each male a weighting factor α ( L ), where α is a decreasing function of the male's expected adult life span, L (see the electronic supplementary material).
Life histories . Each individual, male or female, passes through a period of nursing, weaned dependency, independent juvenility, fertility or eligibility, and females also reach an age of frailty. Individuals of age 0 to τ 0 are still nursing, where τ 0 is the age of weaning, and individuals of age τ 0 to τ 1 ( L ) are weaned, but still dependent, where τ 1 ( L ) is the age of independence and is a function of expected adult life span, L . Age at maturity, τ 2 ( L ), is also a function of expected adult life span. This is the age at which females become fertile and eligible to conceive. Females of age τ 2 ( L ) to τ 3 are fertile, where τ 2 ( L ) is the age of female maturity and τ 3 is the end of fertility. Post-fertile females of age τ 3 to τ 4 ( L ) are eligible to grandmother, where τ 4 ( L ) is the age of frailty. Males of age σ 1 to σ 2 can compete for paternities, where σ 1 and σ 2 specify the beginning and end of eligibility, respectively.
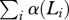
Grandmothering . We consider a more generalized form of allomaternal care than literal grandmothering, in which females who are eligible to grandmother can assume care of any weaned dependent in the population, not only direct matrilineal descendants. This generalization weakens grandmother effects, but it allows us to easily rewrite our agent-based model as a deterministic system, since we do not keep track of matrilineal lineages.
For convenience, we still use the term grandmothering to refer to general transfers of dependents between fertile and post-fertile females. In our model, grandmothering occurs whenever a female who is no longer fertile and has no current dependent adopts a weaned dependent from a female of fertile age, freeing the fertile female for another conception. When a grandmother adopts a child, she functions thereafter as though she were the child's mother.
We further weaken potential grandmother effects by restricting eligibility to females who are past the fertile ages, but have not reached frailty, with the age of frailty varying as a function of expected adult life span (min {2 L , 75}). Since the end of fertility is fixed at 45 years (as suggested by the empirical pattern for humans and great apes), the frailty constraint ensures that there are no eligible grandmothers when expected adult life span is less than 22.5 years. At L = 23, only 45 year olds without dependents are eligible, so less than 1 per cent of the caring females are eligible to grandmother. Our frailty constraint reduces the effects of having females grandmother at unreasonably old ages (see the electronic supplementary material for further discussion).
The deterministic system (see the electronic supplementary material) can be much more efficiently simulated than the agent-based model, allowing us to investigate a wide range of scenarios rapidly. The conclusions of our analysis appear sufficiently general and robust that they should hold for a more sophisticated model that tracks separate lineages.
Parameter estimates for the model are summarized in table 1 . See the electronic supplementary material for further discussion.
Model parameters, descriptions and estimated values. (The variable L denotes expected adult life span. See the electronic supplementary material for discussion of parameter values.)
For populations with fixed expected adult life spans, L , we numerically calculated the net growth rates, r , at a steady-state age distribution with and without grandmothering and plotted the results in figure 1 . Peaks on this plot correspond to local optima of reproductive output for females. They are not population-level equilibria since the effects of male trade-offs are absent, but they would be equilibria in a one-sex version of our model.
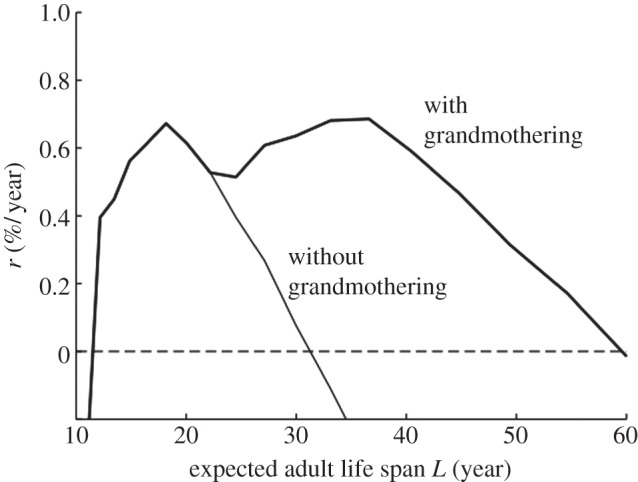
Female reproductive output as a function of expected adult life span. Plots of net growth rate, r , versus expected adult life span, L , for populations with and without grandmothering. (In this simulation, the time step, Δ t , was taken to be 1/12 years to generate a smoother plot. Such a small time step proved to be too computationally demanding for other simulations.)
Figure 1 shows that except at the optimum, L = 18.2, the population grows at a rate of less than 0.7 per cent per year. Since females cannot grandmother before age τ 3 = 45, or after τ 4 ( L ) = 2 L , none are eligible to grandmother in populations with L ≤ 22.5, so growth rates with and without grandmothering coincide in this region. Without grandmothering, the growth rate decreases past L = 18.2 until it falls below 0, at which point the population cannot sustain itself. The decrease in r results from increasing ages at first birth, decreasing fertile periods and increasing ages of independence; but with grandmothering, the growth rate rises gradually from L = 22.5 to 37 and stays above 0 up to approximately L = 60 because mothers can transfer dependents to grandmother care, allowing birth intervals to remain steady (see the electronic supplementary material, for further analysis).
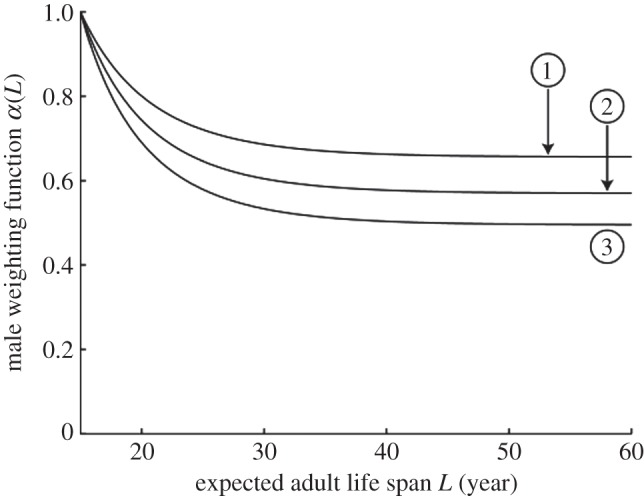
Male trade-offs also play a role in determining population equilibria in our model (see the electronic supplementary material). We consider three male trade-off functions shown in figure 2 . The competitiveness of males decreases with L .
Three male weighting functions, α ( L ), versus expected adult life span, L .
With the female trade-offs that arise from the parameter values in table 1 and give the female reproductive output plotted in figure 1 , the male trade-off curves in figure 2 push the geometric mean of expected adult life spans in the population to equilibrium values of (1) 25.4, (2) 24.9, and (3) 24.6 years in the absence of grandmothering (see the electronic supplementary material, figure S2). (We use the geometric mean, since we represent expected adult life spans on a logarithmic scale as discussed in the electronic supplementary material.) To investigate the effect of grandmothering, we start at those equilibria and introduce grandmothering. Figure 3 shows the evolution of the geometric mean of expected adult life spans in the population from equilibria without grandmothering to equilibria with grandmothering. Sexual conflict pushes the population to an equilibrium L that is an inevitable compromise, neither sex achieving the L that would maximize its reproductive output in the absence of net effects on the opposite sex.
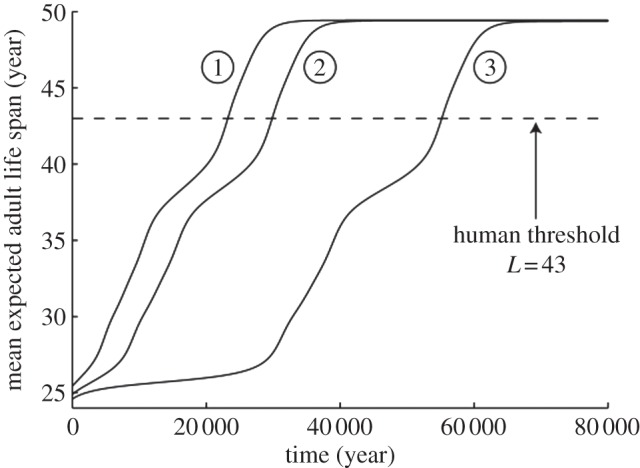
Evolution of populations from lower to higher expected adult life spans in the presence of grandmothering. The starting points (1) 25.4, (2) 24.9 and (3) 24.6 years correspond to equilibria without grandmothering of the three male trade-off curves in figure 2 . Mean expected adult life spans over the population converge to (1) 49.43, (2) 49.40 and (3) 49.37 years in the presence of grandmothering. The population crosses the human threshold of L = 43 within (1) 24 000, (2) 30 000 and (3) 56 000 years.
3. Discussion
Our model population moves from chimpanzee-like life spans into the human longevity range as grandmothers allow mothers to have their next baby sooner without reducing the survival chances of previous offspring. Longer adult life spans (resulting from lower adult mortality) always confer an increased chance of living through the fertile years. But longer-lived females have later ages of first birth and their longer-lived offspring remain dependent to older ages (as holds for mammals generally [ 26 ]). Without grandmothering, the longevity that maximizes female lifetime reproductive success depends on the sum of these effects. Grandmothering alters the equilibrium. Grandmothering also alters the longevity that maximizes male reproductive success (see the electronic supplementary material for further discussion). Our simulations show that by altering the payoffs for both sexes, even weak grandmothering drives the evolution of longevity from an ape-like value into the human range.
We have made no assumptions about sex-biased dispersal, an issue often raised as a problem for the Grandmother Hypothesis (see the electronic supplementary material). Rather than grandmothers helping only their own daughters, our model distributes grandmothering to any eligible dependents in the population. It may seem that this would not only weaken selection for grandmothering, but undercut it altogether. Those with shorter life spans spend little or no time grandmothering and have higher rates of offspring production. If females with lower L free-ride on grandmothering supplied by others, this should halt the spread of grandmothering and the evolution of increasing life spans.
Selection will lead to increased longevity only if grandmothers disproportionately favour their own fitness. Our simulations show that they do, even without a bias toward daughters. Increasing L raises the number of grandmothering years and grandmothering gives greater benefits to females with higher L because their offspring probably have higher L as well. The latter point matters because offspring with higher L have higher survival and are dependent longer, making them more likely to be adopted. In addition, grandmothers take oldest dependents first, disproportionately accepting those with higher L . Although adoption does not benefit the dependents themselves, it does benefit their mothers. This differential benefit that mothers with higher L gain from grandmothering drives the evolution of increased longevity.
Examination of our model populations underlines how little grandmothering it takes to produce the longevity change. At the initial ape-like equilibrium, birth intervals are just over 5 years—shorter than those of orangutans, longer than gorillas' and close to the empirical value for chimpanzees [ 27 ]. At the grandmothering equilibrium where L = 49, age at independence is 8.2. Since children are eligible to leave mothers for grandmothers at 2 years, this nursing period plus a year to conceive and deliver the next baby would make birth intervals 3 years—again close to the empirical value for humans [ 1 ]. But all mothers could do that only if grandmothers cared for children during 6.2 of the 8.2 years of dependency. Given our assumptions that only females past 45 years are eligible and can only care for one dependent at a time, there are not enough grandmothers to make birth intervals that short.
With our assumptions, eligible grandmothers initially make up less than 1 per cent of caring females, but that proportion steadily increases to 43 per cent at the grandmothering equilibrium. In other words, at the equilibrium age distribution, grandmothers care for 43 per cent of the dependent years (43% of 8.2 = 3.5), leaving mothers responsible for the remaining 4.7 of the 8.2 dependent years. If mothers hand off dependent juveniles at an average age of 4.7 years, and then take another year to conceive and deliver the next baby, their intervals are 5.7 years—even longer than those at the initial non-grandmothering equilibrium. Although grandmothering shortens intervals, the weak grandmothering in this model can push the population to human longevities without the very short birth intervals that distinguish humans from other great apes.
Of course in the real world mothers get help from sources not included in our model [ 28 ]. For example, fathers sometimes help [ 29 ] as do older siblings [ 30 ], and stronger grandmother effects could also play a role. However, in our model, we have only allowed females past the age of 45 years to grandmother and diluted the effects by distributing their help throughout the population, restricting subsidies to one dependent at a time, and ignoring probable economies of scale and the decreasing amounts of help required by older dependents. We have fixed the end of fertility at 45 years on grounds that this feature is little changed in humans compared with the other great apes. We leave investigation of ‘why 45?’ to future work, here demonstrating only that given that end to fertility, grandmothering can account for the evolution of increased longevity.
Other hypotheses for the evolution of human longevity appeal to our large brains [ 31 ]. Kaplan et al .'s [ 32 , 33 ] embodied capital model links the evolution of larger human brains to increased skill learning that allowed ancestral hunters to be productive enough to provision their mates and offspring. Kaplan et al . argue that these skills take a long time to learn, with benefits fully realized only well into adulthood. This increases the payoffs for living to older ages and so favours increased somatic maintenance and longer life spans. Our model, in contrast, assumes nothing about the larger brains, hunting, skill learning or pair bonds that distinguish modern humans from the other great apes. It shows that very weak grandmothering can move life spans from the great ape to the human range without any of those features. Our model is also silent on social capacities that others have associated with reliance on allomaternal care across the mammals including humans [ 28 , 34 ]. Hrdy's [ 28 ] synthesis flags especially important selection pressures on both mothers and offspring that accompanied the ancestral switch from an ape pattern of independent rearing to the human pattern of reliance on help. Grandmothers were the probable initial source of that rearing help. As our model shows, selection for grandmothering alone could have propelled the evolution of our post-menopausal longevity, amplifying interdependencies and setting the social context for many other features that subsequently evolved in our lineage.
Acknowledgements
We thank Earle Keefe for compiling data on alpha male chimpanzee tenures, Fred Adler, Adrian Bell, Brett Kennedy, and two anonymous reviewers for clarifying suggestions, and the National Science Foundation, grant no. 0717886, and the Australian Research Council, Discovery Early Career Research Award for support.
- 1. Robson S. L., van Schaik C. P., Hawkes K. 2006. The derived features of human life history. In The evolution of human life history (eds Hawkes K., Paine R.), pp. 17–44 Santa Fe, NM: SAR Press [ Google Scholar ]
- 2. Finch C. E. 2010. Evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proc. Natl Acad. Sci. USA 107, 1718–1724 10.1073/pnas.0909606106 ( doi:10.1073/pnas.0909606106 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Howell N. 1979. Demography of the Dobe !Kung. New York, NY: Academic Press [ Google Scholar ]
- 4. Hill K., Hurtado A. M. 1996. Ache life history: the ecology and demography of a foraging people. New York, NY: Aldine de Gruyter [ Google Scholar ]
- 5. Blurton Jones N. G., Hawkes K., O'Connell J. F. 2002. Antiquity of postreproductive life: are there modern impacts on hunter–gatherer postreproductive life spans? Am. J. Hum. Biol. 14, 184–205 10.1002/ajhb.10038 ( doi:10.1002/ajhb.10038 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Hawkes K., Blurton Jones N. G. 2005. Human age structures, paleodemography, and the grandmother hypothesis. In Grandmotherhood: the evolutionary significance of the second half of female life (eds Voland E., Chasiotis A., Schiefenhovel W.), pp. 118–140 New Brunswick, Canada: Rutgers University Press [ Google Scholar ]
- 7. Hamilton W. D. 1966. The moulding of senescence by natural selection. J. Theoret. Biol. 12, 12–45 10.1016/0022-5193(66)90184-6 ( doi:10.1016/0022-5193(66)90184-6 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Hawkes K., O'Connell J. F., Blurton Jones N. G. 1989. Hardworking Hadza grandmothers. In Comparative socioecology: the behavioural ecology of humans and other mammals (eds Standen V., Foley R. A.), pp. 341–366 Oxford, UK: Blackwell Scientific [ Google Scholar ]
- 9. Hawkes K., O'Connell J. F., Blurton Jones N. G., Alvarez H. P., Charnov E. L. 1998. Grandmothering, menopause, and the evolution of human life histories. Proc. Natl Acad. Sci. USA 95, 1336–1339 10.1073/pnas.95.3.1336 ( doi:10.1073/pnas.95.3.1336 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. O'Connell J. F., Hawkes K., Blurton Jones N. G. 1999. Grandmothering and the evolution of Homo erectus . J. Hum. Evol. 36, 461–485 10.1006/jhev.1998.0285 ( doi:10.1006/jhev.1998.0285 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Hawkes K. 2003. Grandmothers and the evolution of human longevity. Am. J. Hum. Biol. 15, 380–400 10.1002/ajhb.10156 ( doi:10.1002/ajhb.10156 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Voland E., Chasiotis A., Schiefenhovel W. (eds) 2005. Grandmotherhood: the evolutionary significance of the second half of female life. New Brunswick, Canada: Rutgers University Press [ Google Scholar ]
- 13. Sear R., Mace R. 2008. Who keeps children alive? A review of the effects of kin on child survival. Evol. Hum. Behav. 29, 1–18 10.1016/j.evolhumbehav.2007.10.001 ( doi:10.1016/j.evolhumbehav.2007.10.001 ) [ DOI ] [ Google Scholar ]
- 14. Sear R., Coall D. 2011. How much does family matter? Cooperative breeding and the demographic transition. Pop. Dev. Rev. 37, 81–112 10.1111/j.1728-4457.2011.00379.x ( doi:10.1111/j.1728-4457.2011.00379.x ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Hill K., Hurtado A. M. 2009. Cooperative breeding in South American hunter–gatherers. Proc. R. Soc. B 276, 3863–3870 10.1098/rspb.2009.1061 ( doi:10.1098/rspb.2009.1061 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Williams G. C. 1957. Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411 10.2307/2406060 ( doi:10.2307/2406060 ) [ DOI ] [ Google Scholar ]
- 17. Paul A. 2005. Primate predispositions for human grandmaternal behavior. In Grandmotherhood: the evolutionary significance of the second half of female life (eds Voland E., Chasiotis A., Schiefenhovel W.), pp. 21–37 New Brunswick, Canada: Rutgers University Press [ Google Scholar ]
- 18. Walker M. L., Herndon J. G. 2008. Menopause in nonhuman primates? Biol. Reprod. 79, 398–406 10.1095/biolreprod.108.068536 ( doi:10.1095/biolreprod.108.068536 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Rogers A. R. 1993. Why menopause? Evol. Ecol. 7, 406–420 10.1007/bf01237872 ( doi:10.1007/bf01237872 ) [ DOI ] [ Google Scholar ]
- 20. Shanley D. P., Sear R., Mace R., Kirkwood T. B. L. 2007. Testing evolutionary theories of menopause. Proc. R. Soc. B 274, 2943–2949 10.1098/rspb.2007.1028 ( doi:10.1098/rspb.2007.1028 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Cant M. A., Johnstone R. A. 2008. Reproductive conflict and the separation of reproductive generations in humans. Proc. Natl Acad. Sci. USA 105, 5332–5336 10.1073/pnas.0711911105 ( doi:10.1073/pnas.0711911105 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Lee R. D. 2003. Rethinking the evolutionary theory of aging: transfers, not births, shape social species. Proc. Natl Acad. Sci. USA 100, 9637–9642 10.1073/pnas.1530303100 ( doi:10.1073/pnas.1530303100 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Kachel A. F., Premo L. S., Hublin J. J. 2011. Grandmothering and natural selection. Proc. R. Soc. B 278, 384–391 10.1098/rspb.2010.1247 ( doi:10.1098/rspb.2010.1247 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Hawkes K., Kim P. S., Kennedy B., Bohlender R., Hawks J. A. 2011. Reappraisal of grandmothering and natural selection. Proc. R. Soc. B 278, 1936–1938 10.1098/rspb.2010.2720 ( doi:10.1098/rspb.2010.2720 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Kachel A. F., Premo L. S., Hublin J. J. 2011. Grandmothering and natural selection revisited reply. Proc. R. Soc. B 278, 1939–1941 10.1098/rspb.2011.0472 ( doi:10.1098/rspb.2011.0472 ) [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Charnov E. L. 1993. Life history invariants: some explorations of symmetry in evolutionary ecology. Oxford, UK: Oxford University Press [ Google Scholar ]
- 27. Knott C. 2001. Female reproductive ecology of the apes: implications for human evolution. In Reproductive ecology and human evolution (ed. Ellison P. T.), pp. 429–463 New York, NY: Aldine de Gruyter [ Google Scholar ]
- 28. Hrdy S. B. 2009. Mothers and others: the evolutionary origins of mutual understanding. Cambridge, MA: Belknap Press of Harvard University Press [ Google Scholar ]
- 29. Grey P., Anderson K. G. 2010. Fatherhood: evolution and human paternal behavior. Cambridge, MA: Harvard University Press [ Google Scholar ]
- 30. Kramer K. 2011. The evolution of human parental care and recruitment of juvenile help. Trends Ecol. Evol. 26, 533–540 10.1016/j.tree.2011.06.002 ( doi:10.1016/j.tree.2011.06.002 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Isler K., van Schaik C. P. 2009. The expensive brain: a framework for explaining evolutionary changes in brain size. J. Hum. Evol. 57, 392–400 10.1016/j.jhevol.2009.04.009 ( doi:10.1016/j.jhevol.2009.04.009 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Kaplan H., Hill K., Lancaster J., Hurtado A. M. 2000. A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185 ( doi:10.1002/1520-6505(2000)9:4<156::AID-EVAN5>3.0.CO;2-7 ) [ DOI ] [ Google Scholar ]
- 33. Kaplan H., Gurven M., Winking J., Hooper P., Stieglitz J. 2010. Learning, menopause and the human adaptive complex. Ann. NY Acad. Sci. 1204, 30–42 10.1111/j.1749-6632.2010.05528.x ( doi:10.1111/j.1749-6632.2010.05528.x ) [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Burkart J., van Schaik C. 2010. Cognitive consequences of cooperative breeding in primates? Anim. Cogn. 13, 1–19 10.1007/s10071-009-0263-7 ( doi:10.1007/s10071-009-0263-7 ) [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (402.7 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Grandmother Hypothesis, The
- Reference work entry
- First Online: 01 January 2021
- pp 3499–3503
- Cite this reference work entry

- Mirkka Lahdenperä 3 ,
- Antti O Tanskanen 4 , 6 &
- Mirkka Danielsbacka 4 , 5
222 Accesses
Altriciality life span hypothesis ; Mother hypothesis ; Stopping-early hypothesis
The grandmother hypothesis states that the long post-reproductive life span in human females would have evolved because women were able to gain more fitness by investing in their adult offspring and grand-offspring rather than by reproducing until old age. Because of this fitness benefit, selection would have favored a longer post-reproductive life span during human evolution.
Introduction
In most animals, reproductive and somatic senescence occurs at the same time as a part of the gradual decline in overall physical condition with age. In few species, however, the reproductive functions show an abrupt deterioration well before other body functions, leading to a total loss of fertility during middle age and subsequent post-reproductive life span of several decades. So far, the most convincing evidence from menopause and long post-reproductive life span comes from a few whale species,...
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Cant, M. A., & Johnstone, R. A. (2008). Reproductive conflict and the separation of reproductive generations in humans. Proceedings of the National Academy of Sciences, USA, 105 , 5332–5336. https://doi.org/10.1073/pnas.0711911105 .
Article Google Scholar
Caspari, R., & Lee, S. H. (2004). Older age becomes common late in human evolution. Proceedings of the National Academy of Sciences, USA, 101 , 10895–10900. https://doi.org/10.1073/pnas.0402857101 .
Coall, D. A., & Hertwig, R. (2010). Grandparental investment: Past, present, and future. Behavioral and Brain Sciences, 33 (1), 1–19. https://doi.org/10.1017/S0140525X09991105 .
Article PubMed Google Scholar
Croft, D. P., Brent, L. J. N., Franks, D. W., & Cant, M. A. (2015). The evolution of prolonged life after reproduction. Trends in Ecology & Evolution, 30 (7), 407–416. https://doi.org/10.1016/j.tree.2015.04.011 .
Croft, D. P., Johnstone, R. A., Ellis, S., Nattrass, S., Franks, D. W., Brent, L. J., et al. (2016). Reproductive conflict and the evolution of menopause in killer whales. Current Biology, 27 (2), 298–304. https://doi.org/10.1016/j.cub.2016.12.015 .
Ellis, S., Franks, D. W., Nattrass, S., Cant, M. A., Bradley, D. L., Giles, D., et al. (2018). Postreproductive lifespans are rare in mammals. Ecology and Evolution, 8 (5), 2482–2494. https://doi.org/10.1002/ece3.3856 .
Article PubMed PubMed Central Google Scholar
Fairbanks, L. A., & Mcguire, M. T. (1986). Age, reproductive value, and dominance-related behavior in vervet monkey females – crossgenerational influences on social relationships and reproduction. Animal Behaviour, 34 (6), 1710–1721. https://doi.org/10.1016/S0003-3472(86)80258-5 .
Hamilton, W. D. (1964). The genetical evolution of social behaviour I and II. Journal of Theoretical Biology, 7 (1), 1–16, 17–52. https://doi.org/10.1016/0022-5193(64)90038-4 , https://doi.org/10.1016/0022-5193(64)90039-6 .
Hawkes, K. (2003). Grandmothers and the evolution of human longevity. American Journal of Human Biology, 15 (3), 380–400. https://doi.org/10.1002/ajhb.10156 .
Hawkes, K., Rogers, A. R., & Charnov, E. L. (1995). The male’s dilemma: Increased offspring production is more paternity to steal. Evolutionary Ecology, 9 (6), 662–677. https://doi.org/10.1007/BF01237661 .
Hawkes, K., O’Connell, J. F., & Blurton Jones, N. G. (1997). Hadza women’s time allocation, offspring provisioning, and the evolution of long postmenopausal life spans. Current Anthropology, 38 (4), 551–557.
Hawkes, K., O’Connell, J. F., Blurton Jones, N. G., Alvarez, H., & Charnov, E. L. (1998). Grandmothering, menopause, and the evolution of human life histories. Proceedings of the National Academy of Sciences, USA, 95 (3), 1336–1339.
Kaplan, H., Hill, K., Lancaster, J., & Hurtado, M. (2000). A theory of human life history evolution: Diet, intelligence, and longevity. Evolutionary Anthropology, 9 (4), 156–185. https://doi.org/10.1002/1520-6505(2000)9:4<156::AID-EVAN5>3.0.CO;2-7 .
Kim, P. S., Coxworth, J. E., & Hawkes, K. (2012). Increased longevity evolves from grandmothering. Proceedings of the Royal Society B: Biological Sciences, 279 , 4880–4884. https://doi.org/10.1098/rspb.2012.1751 .
Lahdenperä, M., Lummaa, V., Helle, S., Tremblay, M., & Russell, A. F. (2004). Fitness benefits of prolonged post-reproductive lifespan in women. Nature, 428 , 178–181. https://doi.org/10.1038/nature02367 .
Lahdenperä, M., Gillespie, D. O., Lummaa, V., & Russell, A. F. (2012). Severe intergenerational reproductive conflict and the evolution of menopause. Ecology Letters, 15 (11), 1283–1290. https://doi.org/10.1111/j.1461-0248.2012.01851.x .
Lahdenperä, M., Mar, K. U., & Lummaa, V. (2016). Nearby grandmother enhances calf survival and reproduction in Asian elephants. Scientific Reports, 6 , 27213. https://doi.org/10.1038/srep27213 .
Møller, A. P., Fincher, C. L., & Thornhill, R. (2009). Why men have shorter lives than women: Effects of resource availability, infectious disease, and senescence. American Journal of Human Biology, 21 (3), 357–364. https://doi.org/10.1002/ajhb.20879 .
Morton, R. A., Stone, J. R., & Singh, R. S. (2013). Mate choice and the origin of menopause. PLoS Computational Biology, 9 (6), e1003092. https://doi.org/10.1371/journal.pcbi.1003092 .
Pavelka, M. S. M., Fedigan, L. M., & Zohar, S. (2002). Availability and adaptive value of reproductive and postreproductive Japanese macaque mothers and grandmothers. Animal Behaviour, 64 (3), 407–414. https://doi.org/10.1006/anbe.2002.3085 .
Penn, D. J., & Smith, K. R. (2007). Differential fitness costs of reproduction between the sexes. Proceedings of the National Academy of Sciences, USA, 104 (2), 553–558. https://doi.org/10.1073/pnas.0609301103 .
Sear, R., & Coall, D. (2011). How much does family matter? Cooperative breeding and the demographic transition. Population and Development Review, 37 (Supplement), 81–112. https://doi.org/10.1111/j.1728-4457.2011.00379.x .
Sear, R., & Mace, R. (2008). Who keeps children alive? A review of the effects of kin on child survival. Evolution and Human Behavior, 29 , 1–18. https://doi.org/10.1016/j.evolhumbehav.2007.10.001 .
Tanskanen, A. O., & Danielsbacka, M. (2017). Multigenerational effects on children’s cognitive and socioemotional outcomes: A within-child investigation. Child Development , 88 . https://doi.org/10.1111/cdev.12968 .
Tanskanen, A. O., & Danielsbacka, M. (2018). Intergenerational family relations. An evolutionary social science approach . London: Routledge.
Book Google Scholar
Tuljapurkar, S. D., Puleston, C. O., & Gurven, M. D. (2007). Why men matter: Mating patterns drive evolution of human lifespan. PLoS One, 2 (8), e785. https://doi.org/10.1371/journal.pone.0000785 .
Voland, E., & Beise, J. (2002). Opposite effects of maternal and paternal grandmothers on infant survival in historical Krummhörn. Behavioral Ecology and Sociobiology, 52 (6), 435–443.
Voland, E., Chasiotis, A., & Schiefenhovel, W. (Eds.). (2005). Grandmotherhood: The evolutionary significance of the second half of female life . New Brunswick: Rutgers University Press.
Google Scholar
Williams, G. C. (1957). Pleiotropy, natural selection and the evolution of senescence. Evolution, 11 , 398–411. https://doi.org/10.1111/j.1558-5646.1957.tb02911.x .
Wood, J. W., O’Connor, K. A., Holman, D. J., Brindle, E., Barsom, S. H., & Grimes, M. A. (2000). The evolution of menopause by antagonistic pleiotropy. Homo, 51 , S149.
Download references
Author information
Authors and affiliations.
Department of Biology, University of Turku, Turku, Finland
Mirkka Lahdenperä
Department of Social Research, University of Turku, Turku, Finland
Antti O Tanskanen & Mirkka Danielsbacka
Population Research Institute of Finland, Helsinki, Finland
Mirkka Danielsbacka
Population Research Institute of Finland, University of Helsinki, Helsinki, Finland
Antti O Tanskanen
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Antti O Tanskanen .
Editor information
Editors and affiliations.
Department of Psychology, Oakland University, Rochester, MI, USA
Todd K Shackelford
Viviana A Weekes-Shackelford
Section Editor information
Rights and permissions.
Reprints and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this entry
Cite this entry.
Lahdenperä, M., Tanskanen, A.O., Danielsbacka, M. (2021). Grandmother Hypothesis, The. In: Shackelford, T.K., Weekes-Shackelford, V.A. (eds) Encyclopedia of Evolutionary Psychological Science. Springer, Cham. https://doi.org/10.1007/978-3-319-19650-3_2340
Download citation
DOI : https://doi.org/10.1007/978-3-319-19650-3_2340
Published : 22 April 2021
Publisher Name : Springer, Cham
Print ISBN : 978-3-319-19649-7
Online ISBN : 978-3-319-19650-3
eBook Packages : Behavioral Science and Psychology Reference Module Humanities and Social Sciences Reference Module Business, Economics and Social Sciences
Share this entry
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

IMAGES
COMMENTS
The grandmother hypothesis is a hypothesis to explain the existence of menopause in human life history by identifying the adaptive value of extended kin networking. It builds on the previously postulated "mother hypothesis" which states that as mothers age, the costs of reproducing become greater, and energy devoted to those activities would be better spent helping her offspring in their ...
Feb 7, 2019 · The first hard evidence for the grandmother hypothesis was gathered by Kristen Hawkes, an anthropologist at the University of Utah who was studying the Hadza people, a group of hunter-gatherers in ...
Key Words: Grandmother hypothesis, Evolution, Aging and cognition, Non-human primates. Introduction. Women may enjoy many years of life after their childbearing careers have ended. One explanation as to how a long life span past fertility arose in our species is the grandmother hypothesis. This hypothesis holds that, in response to ecological ...
Jun 1, 2020 · Among our closest living evolutionary cousins, mothers rear each offspring to weaning when it feeds itself. Human youngsters always need others' help (e.g. [1,3,6–10]). The grandmother hypothesis specifies the socioecological context that likely propelled the evolution of this distinctive human life history.
Mar 11, 2004 · The grandmother effect. Kristen Hawkes 1 ... Charnov, E. L. Life History Invariants: Some Explorations of Symmetry in Evolutionary Ecology (Oxford Univ. Press, 1993). Google Scholar
Dec 12, 2012 · Human age structures, paleodemography, and the grandmother hypothesis. In Grandmotherhood: the evolutionary significance of the second half of female life (eds Voland E., Chasiotis A., Schiefenhovel W.), pp. 118–140 New Brunswick, Canada: Rutgers University Press [Google Scholar]
Jan 1, 2021 · The grandmother hypothesis presents a compelling evolutionary explanation for life after menopause, particularly in human females. As it stands, there is good evidence for the hypothesis that human life spans expanded beyond the limitations of oocyte production and maintenance in order to better facilitate cooperative breeding with kin.
Nov 1, 2013 · A grandmother hypothesis may explain why greater longevity evolved in the authors' lineage while female fertility still ends at ancestral ages, and has implications for the evolution of a wide array of human features. Women and female great apes both continue giving birth into their forties, but not beyond. However humans live much longer than other apes do. Even in hunting and gathering ...
Jan 1, 2022 · While the grandmother hypothesis won a large following, it remains a highly controversial topic in evolutionary anthropology since the evidence from contemporary hunter-gather societies and historical data produced mixed results. Some critics have cast doubt on the hypothesis because of several reasons (for a review see Peccei 2001a). One of ...
Jan 1, 2021 · The grandmother hypothesis has gained support from many populations worldwide; however, some studies have shown controversial results. The hypothesis has been tested, for the most part, among populations with natural fertility and mortality, such as historical or hunter-gatherer human populations, but there are also studies in modern human populations living in traditional settings or in ...